Our students are engaged in research spanning a variety of biological systems using basic animal models of disease to clinical approaches for treatment. Departments including Medicine, Neurobiology and the RNA Therapeutics Institute offer training in highly collaborative and supportive communities.

The Xue lab goal is to explore various small RNA tools, including CRISPR/Cas9 platforms, to functionally dissect cancer mutations in mouse models of liver cancer and to identify strategies for disease correction in rare genetic diseases. The Xue lab’s most recent work includes: CRISPR-Cas9 screening to elucidate mechanisms of senescence in liver cancer, repurposing CRISPR-Cas9 for efficient cancer modeling, and base editing for disease correction in familial tyrosinemia. See the research taking place in the Xue lab here: Xue Lab
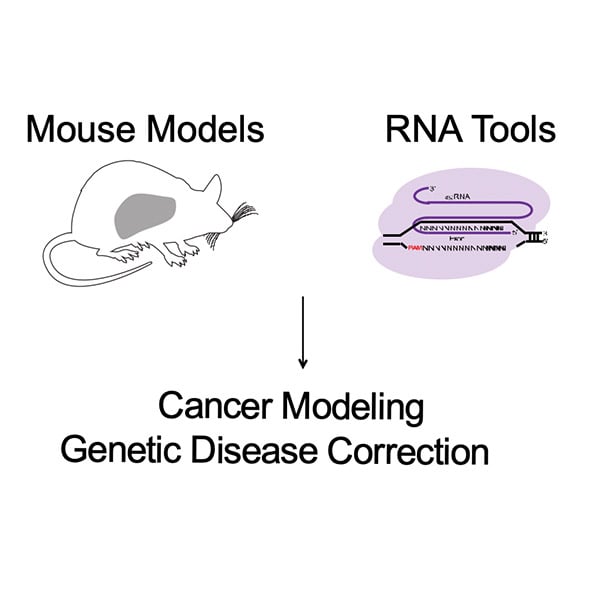

I joined Dr. Wen Xue’slab in 2017. As a rotation student, I explored mechanisms of CRISPR/Cas9 gene editing and helped determine that single guide RNA (sgRNA) can induce alternative exon skipping and large genetic deletions. These findings, published in Genome Biology, demonstrated the potential complications of using CRISPR/Cas9 editing in cancer biology modeling. My thesis work focuses on identifying tractable therapeutic strategies in rare pediatric cancers through exploring mechanism of oncogene addiction and cancer cell differentiation.
Jordan.Smith@umassmed.edu

Dr. Michael A. Brehm, PhD, is an Associate Professor in the Program in Molecular Medicine and a Robert and Sandra Glass Term Chair in Diabetes, as well as serving as a co-director with Dr. Dale L. Greiner, PhD, of a pre-clinical/co-clinical Research Core to advance development and use of models of human specific diseases. The Core is working with The Jackson Laboratory (JAX) and Precision Genetics Center (JPGC) promoting interactions with clinicians and hospitals, access to patient data and samples and comprehensive model assessment necessary for generation of models engrafted with human tumors and immune systems for preclinical testing. The Brehm Lab uses these unique animal models of human immune responses to investigate approaches to downregulate as well as activate the human immune system for treatments of type 1 diabetes and cancer.
Brehm Lab

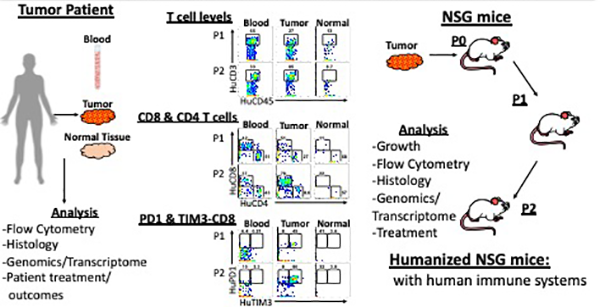
Jane E. Chuprin joined the Brehm Lab in September 2019 with a focus on studying immune responses to pancreatic adenocarcinoma tumor as part of the Avatar Project at UMass Chan. The Avatar Project is such that a patient’s tumor is engrafted into an immunodeficient mouse, and subsequently the patient’s own immune system is also engrafted for a unique model to study how the patient’s own immune system is, or is not, responding its tumor. Different combinations of treatment can then be tested in these humanized mice to see what the best therapy would be for the specific patient and tumor. Jane is focusing on studying these immune responses in depth using multi-parameter flow cytometry (panel of upwards of 30 colors) and other bioinformatic techniques (i.e. CITE-seq). She is also working on investigating a new clinical approach to treatment through intratumoral injections of immunotherapy drugs.
Jane.Chuprin@umassmed.edu

My laboratory has two main research areas: Mechanisms of T cell leukemogenesis and the role of RIP kinases in TNF- and TLR- signaling and cell death. We use the mouse as a model system to interrogate the pathways that control inflammation and to understand the genetic basis of T cell acute lymphoblastic leukemia (T-ALL).
Kelliher Lab
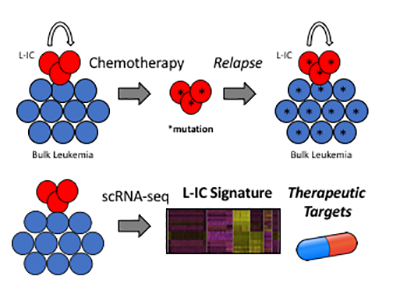

Kevin joined the Kelliher Lab in September 2018. His thesis work aims to better understand leukemia-initiating cells (L-ICs), which are thought to be responsible for T-ALL relapse. By applying a single-cell transcriptomic approach to a murine T-ALL model he aims to identify genetic features of L-ICs amenable to therapeutic intervention in order to improve outcomes for patients with T-ALL.
Kevin.OConnor@umassmed.edu
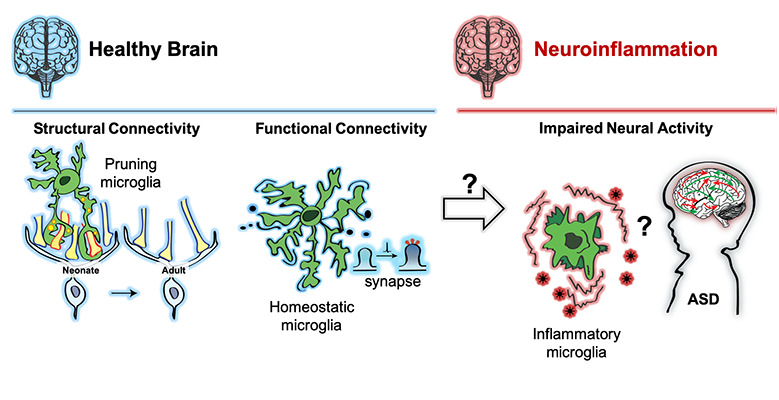

The primary focus of Dr. Schafer’s research is to understand the role of microglia in neural circuit development and plasticity in the healthy and diseased nervous system. Major lines of ongoing research in the lab are aimed at elucidating the molecular mechanisms underlying microglia-synapse interactions, investigating microglial responses to changes in sensory experience and dissecting how microglia contribute to synaptic changes in neurological and psychiatric disorders including multiple sclerosis and autism spectrum disorders. To address these areas, the lab has developed cutting-edge molecular genetic approaches combined with high resolution static and 2-photon live imaging.
Dorothydori.Schafer@umassmed.edu

Philip joined Dori Schafer’s lab in 2016 with an interest in studying the role microglia play in regulating excitatory & inhibitory balance (E/I balance) in the brain. The clinical relevance is exemplified in neuropsychiatric disorders, such as autism spectrum disorders (ASDs), where defects in E/I balance are believed to underlie some of the core clinical features including repetitive behaviors, impaired sociability and increased propensity for seizures. His current projects focus on exploring whether changes in microglial inflammatory state affect E/I balance by studying the contribution of microglial-derived neuroactive cytokines to regulating structural and functional synaptic connectivity. Philip uses genetic mouse tools to dissect the microglial mechanisms and combines this work with an ongoing human study examining inflammatory markers and E/I measurements in adolescents with ASD.
Philip.Feinberg@umassmed.edu
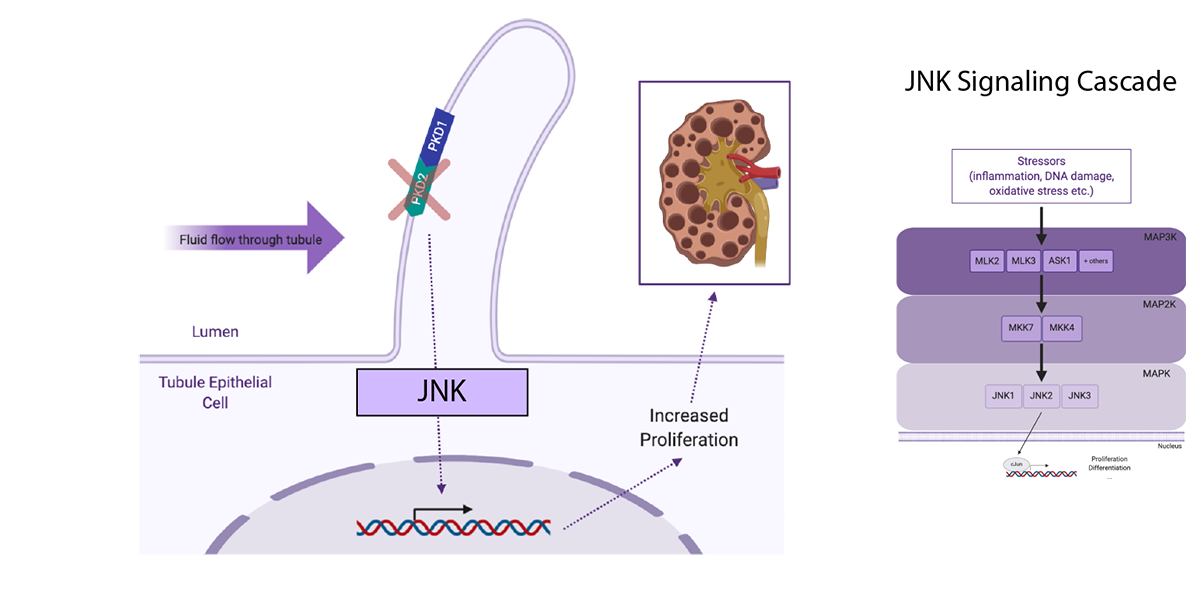

The Pazour lab is interested in the function of the mammalian primary cilium.These organelles play vital roles in the development of mammals and in the etiology of diseases such as polycystic kidney disease and blindness. Our work combines in vitro cell culture studies with mutant mouse models to understand the role of cilia in controlling kidney architecture and formation of the photoreceptor outer segment.
Pazour Lab

Abigail joined the Pazour lab in April of 2017 where she uses mouse models to understand the role of ciliary signaling in autosomal dominant polycystic kidney disease (ADPKD). Cilia on kidney tubule epithelial cells sense and respond to changes in fluid flow and filtrate composition. Mutations in the cilia-localized polycystin proteins (PKD1 and PKD2) induce cyst formation via increased tubule epithelium proliferation and secretion. However, the precise signaling events that lead from polycystin loss to cystic changes remain obscure. Abigail’s thesis project explores the role of MAP kinase signaling in PKD2 knockout mice. Specifically, she finds that c-Jun N-terminal (JNK) signaling contributes to cystic disease in this model.
Abigail.Smith@umassmed.edu


John Harris is an associate professor of dermatology at UMass Chan Medical School, and he is a physician-scientist specialized in the study and treatment of vitiligo. Vitiligo is an autoimmune disease of the skin that destroys the pigment-producing cells, melanocytes, which results in patchy depigmentation. Dr. Harris is the director of the Vitiligo Clinic and Research Center at UMass, where he manages the seamless integration of clinical healthcare, clinical research trials, human translational research, and basic research that all aim to advance the treatment and understanding of vitiligo.
Research Lab

Mike joined the lab in September 2016, and he has always been interested in better understanding how environmental factors influence inflammatory skin diseases. For several years, he studied the clinical phenomenon by which certain household chemicals can trigger and worsen vitiligo; and now Mike studies how environmental chemicals initiate contact dermatitis. Both irritant and allergic contact dermatitis offer safe models of inducible human skin inflammation, which can be studied by pairing non-scarring skin biopsy techniques with powerful scientific assays. Through these studies, Mike aims to discover basic mechanisms that direct the initiation and resolution of human skin inflammation.
Michael.Frisoli@umassmed.edu
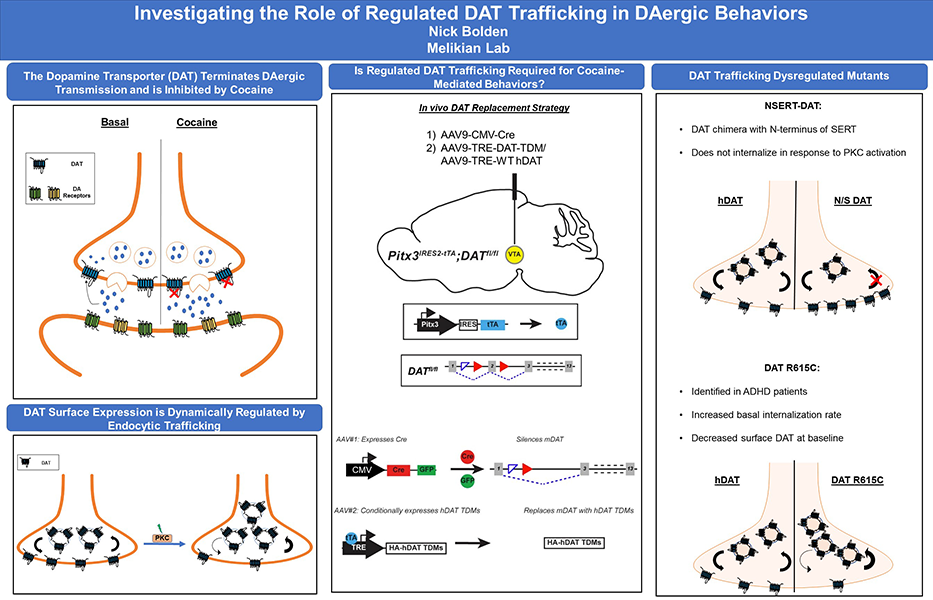

Our laboratory is interested in the molecular mechanisms that regulate presynaptic dopamine reuptake by the dopamine (DA) transporter (DAT). DA neurotransmission is required for executive function, working memory, reward and movement, and DA dysfunction is associated with several neuropsychiatric disorders, including attention-deficit/hyperactivity disorder (ADHD), schizophrenia, addiction and Parkinson’s disease.
Melikian Lab

Dopamine signaling is required for many biological processes including movement, cognition, reward, and motivation. The dopamine transporter (DAT) is expressed pre-synaptically in DAergic terminals and spatiotemporally regulates DAergic transmission and maintains DAergic tone by facilitating reuptake of extracellular dopamine back into the presynaptic neuron, for re-packaging and re-release. DAT is the primary target of therapeutic and addictive psychostimulants, including cocaine, which binds to DAT at the plasma membrane, inhibit DA reuptake, and increase extracellular DA. DAT surface expression is dynamically regulated by endocytic trafficking, and a variety of signaling pathways acutely modulate DAT surface expression. However, it is completely unknown how DAT trafficking impacts DA physiology and DA-dependent behaviors, or if it plays a central role in psychostimulant addiction.
My thesis project aims to address this question by using an intersectional genetic approach that utilizes a combined Cre-lox and Tet-off approach to silence endogenous mDAT protein expression in adolescent mice, and replace wildtype mDAT with trafficking dysregulated hDAT mutants specifically in DAergic neurons. Using this in vivo molecular replacement strategy, I will then test whether DAT trafficking is required for either motor function or cocaine-mediated behaviors.
Nicholas.Bolden@umassmed.edu

Circadian rhythms and photoreception in Drosophila
Research Lab

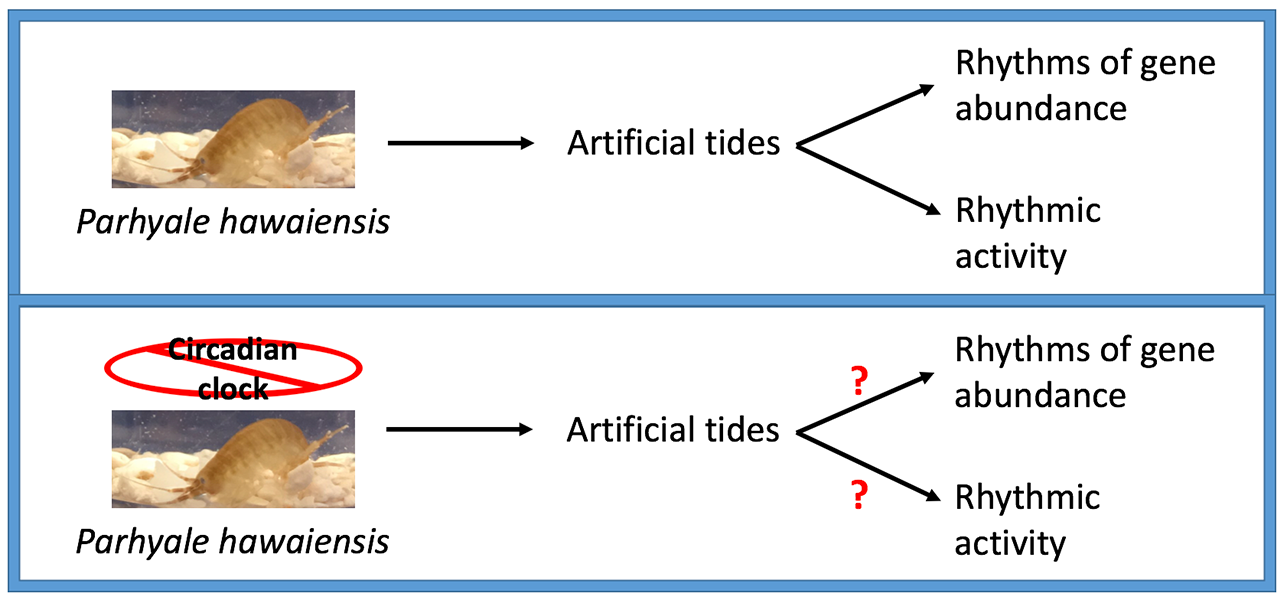
Circatidal rhythms are the biological rhythms entrained by the cyclic rising and falling of the tide. Attempts to identify genetic requirements for circatidal rhythms have been limited to the use of RNAi-knockdown. We designed an approach to entrain and record circatidal rhythms in the CRISPR-Cas9-amenable crustacean Parhyale hawaiensis, and show that P. hawaiensis exhibits free-running rhythms of activity with a period of circa 12.4 hours in constant environmental conditions. In summary, we demonstrate that circatidal rhythms of activity in the crustacean Parhyale hawaiensis fit the key criteria for biological rhythms, and present a novel paradigm for studying the basis of circatidal rhythmicity.
Erica.Kwiatkowski@umassmed.edu
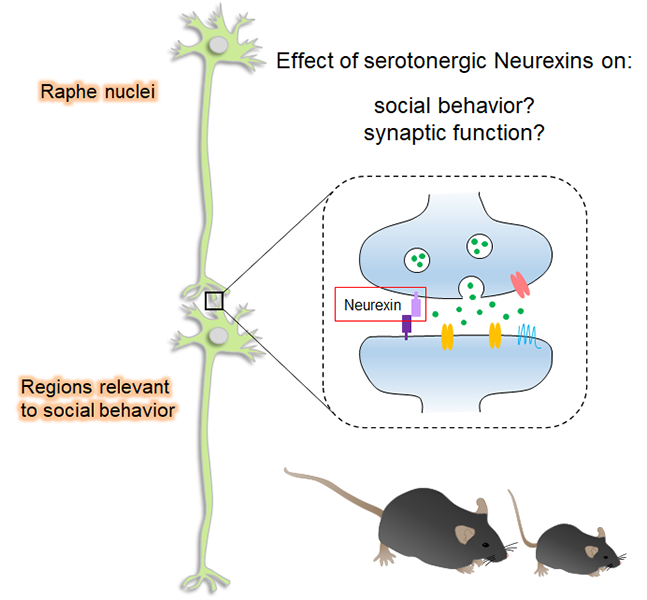

Our research is focused on investigating the relationship between the dysregulation of synaptic function and neuropsychiatric diseases such as schizophrenia and autism spectrum disorders (ASD) by exploring the regulatory mechanism of inhibitory neurons-mediated synaptic transmission. Synapses are a specialized junction of cell-cell contacts that allow for communication between neurons. Synaptic transmission is mediated by neurotransmitters that are released from a presynaptic terminal and act on corresponding receptors on the postsynaptic dendrite. In the mammalian central nervous system, the most excitatory neurons use glutamate as a neurotransmitter while inhibitory neurons use GABA. Neuronal signal processing is mediated by the integration of both excitatory and inhibitory synaptic responses. Therefore, precise regulatory mechanisms must exist to maintain the balance of excitatory and inhibitory synaptic transmission “E/I balance”. It is becoming increasingly clear that neuropsychiatric diseases may arise from the dysregulation of inhibitory neuronal function which leads to a change in the E/I balance. This would suggest that the restoration of inhibitory function can be a possible direction for therapeutic direction.
Research Lab
Amy joined the Futai Lab in 2017 to bring together her behavioral neuroscience background with the lab’s electrophysiological, biochemical, and molecular expertise. Fascinated by neural mechanisms contributing to the output of different behaviors, her thesis work investigates the role of Neurexins, risk genes for Autism Spectrum Disorder, at serotonin synapses in regulating serotonin neurotransmission and social behavior.
Amy.Cheung@umassmed.edu

Study of retroviruses has led to major advances in fundamental biology including the description of the first viruses that cause tumors and the discovery of oncogenes. Description of reverse transcription, the process by which retroviruses make cDNA from the viral RNA genome, transformed understanding of genetic information transfer, though consistent with the Central Dogma of Molecular Biology (see draft with drawing by Francis Crick, below), and created new possibilities for understanding the Origin of Life. In this spirit, we study HIV-1 replication, pathogenesis, and immunity, with the goal of making discoveries of fundamental biological significance.
The Luban Lab

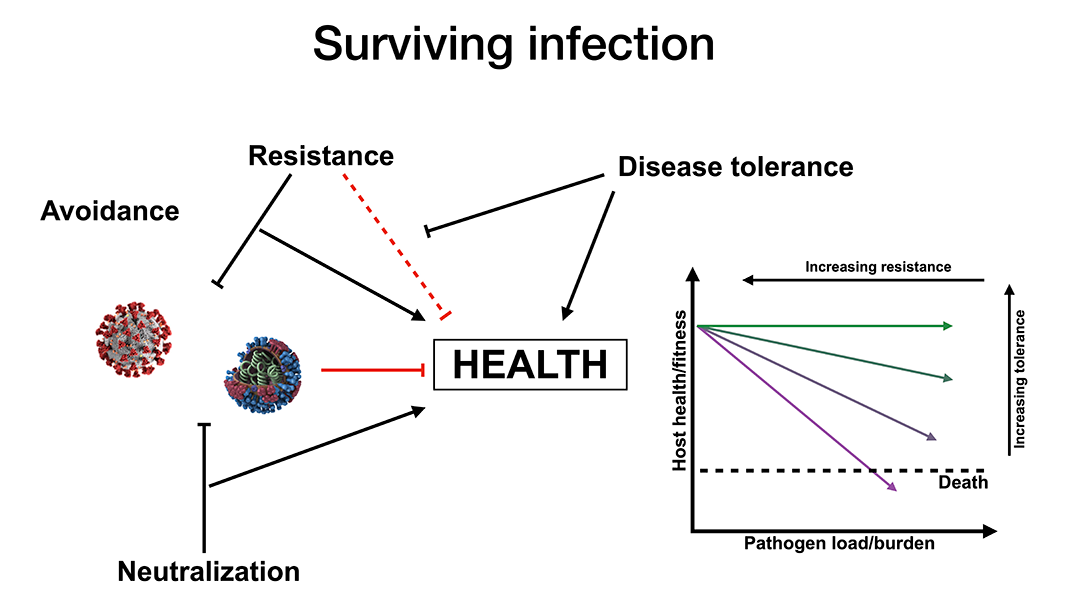
Survival after infection with a pathogenic virus, such as influenza or SARS-CoV-2, requires not only that the immune system control and eliminate the pathogen, but that disease tolerance mechanisms limit tissue damage caused by the pathogen, or by host inflammatory responses. The common thread that ties together my thesis, which includes both a mouse model of epigenetic inheritance (paternal stress affecting offspring development) and a clinical project examining responses to SARS-CoV-2 infection, is this concept of disease tolerance in the context of viral infection.
Noah.Silverstein@umassmed.edu


Anastasia Khvorova, PhD, has more than twenty years of experience developing oligonucleotide technology and therapeutics. She is a professor in the RNA Therapeutics Institute (RTI) and Program in Molecular Medicine at the UMass Chan Medical School (UMass Chan). Her lab brings together hardcore organic and oligonucleotide chemists, RNA biologists, pharmacologists, and bioinformaticians to develop novel approaches and solutions to understanding natural and therapeutic RNA trafficking and delivery. She established the RTI Nucleic Acid Chemistry Center, which provides expertise in RNA chemistry to labs within and outside UMass Chan, and is the only non-profit center in North America capable of synthesizing complex RNAs at scales necessary to support both in vitro and in vivo studies.
Khvorova Lab

Age-related neurodegenerative diseases occur when neurons progressively deteriorate, function abnormally, and die. Patients with neurodegenerative disorders like Alzheimer’s Disease (AD) and Amyotrophic Lateral Sclerosis (ALS) experience impaired cognition or mobility, and cannot live independently. Current therapies manage symptoms—but do not stop progression. This is partially because the root causes of AD and ALS are unclear; and thus, difficult to target. Therapies that modulate common pathways in neurodegeneration— such as lipid homeostasis—rather than the root cause or characteristic feature of each disease (e.g. beta-amyloid in AD) hold promise for modifying progression across multiple neurodegenerative disorders.
Lipid transport in the periphery and central nervous system (CNS) is facilitated by Apolipoprotein E (ApoE). In human plasma and CNS, ApoE levels and isoforms (i.e. E2, E3, E4) are related to AD and ALS onset and progression; and in mice, knocking out ApoE reduces neurodegeneration. Thus, reducing ApoE may be one approach to treat neurodegenerative diseases. Yet, complete loss of ApoE in mice causes atherosclerosis. Given the distinct functions of ApoE “pools” (i.e. systemic and CNS) in lipid transport, pool-specific ApoE reduction may be a more viable therapy than total ApoE silencing. But, is it possible to reduce one ApoE pool without affecting the other, and what is the effect of silencing each ApoE pool on neurodegeneration?
The goal of this project is to use RNA interference (RNAi) technology to determine the effect of silencing each ApoE pool on AD progression. Using GalNAc- and di-siRNAs, we silenced hepatic and CNS ApoE, respectively, and examined the effects on CNS and systemic ApoE pools, and AD phenotypes in mice.
Chantal.Ferguson@umassmed.edu
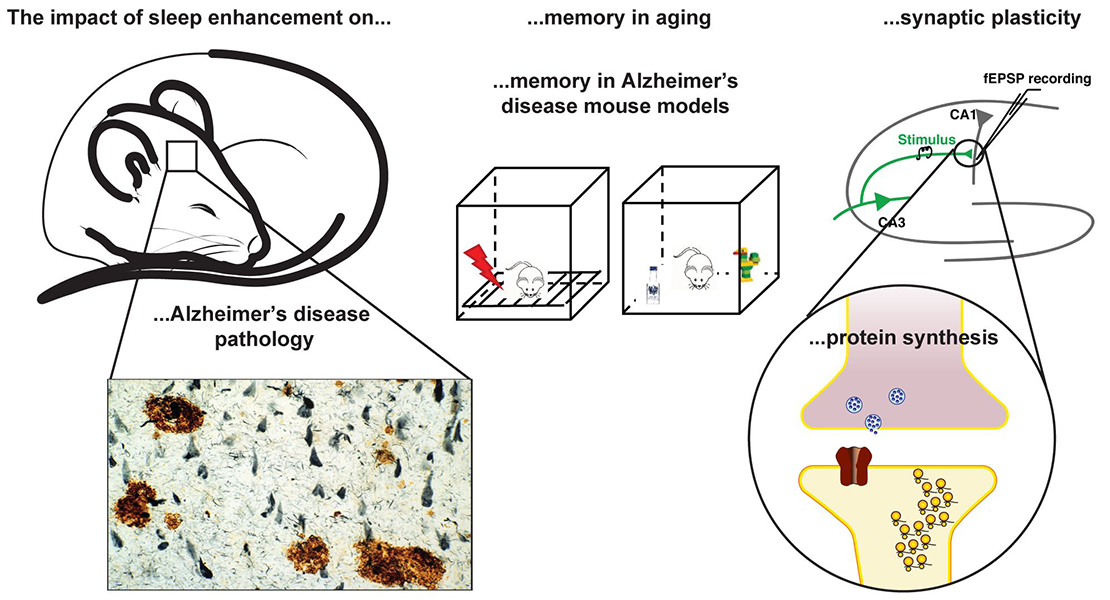

Anaclet Lab is interested in understanding the neuronal circuitries regulating sleep-wake states and the beneficial role of sleep in physiology. We investigate the cellular and circuitry bases of the sleep-promoting medullary parafacial zone. Additionally, using our unique model of sleep-enhancement, we study the beneficial role of sleep in physiology and diseases.
Anaclet Lab Twitter: @anaclet_lab

Sleep deprivation studies indicate that sleep is necessary for memory consolidation, yet sleep deprivation is associated with stress and behaviors resulting in acquisition of additional memory traces that could interfere with these mechanisms. Using a unique model of SWS enhancement in mice, I am investigating the role of deep sleep in memory using two mouse models in which both SWS and cognition are impaired: aging and Alzheimer’s disease. By enhancing the quality and quantity of SWS in aged and Alzheimer’s disease mouse models, I aim to reverse the memory deficits and explore the cellular and molecular basis of sleep-dependent memory consolidation.
Oghomwen.Igiesuorobo@umassmed.edu


The main focus of our lab is to understand which pathways guide beta cell proliferation, to find approaches to make more beta cells, to prevent or treat diabetes. Using knockout and transgenic mice, and human and mouse pancreatic tissue in culture systems, we are following two exciting leads.
Alonso Lab

Rachel joined the Alonso Lab in 2016, and her thesis work focuses on how a cyclin-dependent kinase not only drives beta cell proliferation, but also interacts with the insulin signaling pathway in order to protect against beta cell dedifferentiation, a recently recognized mechanism of beta cell failure in diabetes. She has also worked on a project investigating whether the lab’s interesting concept of increased insulin demand driving beta cell proliferation through UPR activation also occurs in other endocrine tissues, mainly in the parathyroid in CKD.
Rachel.Stamateris@umassmed.edu