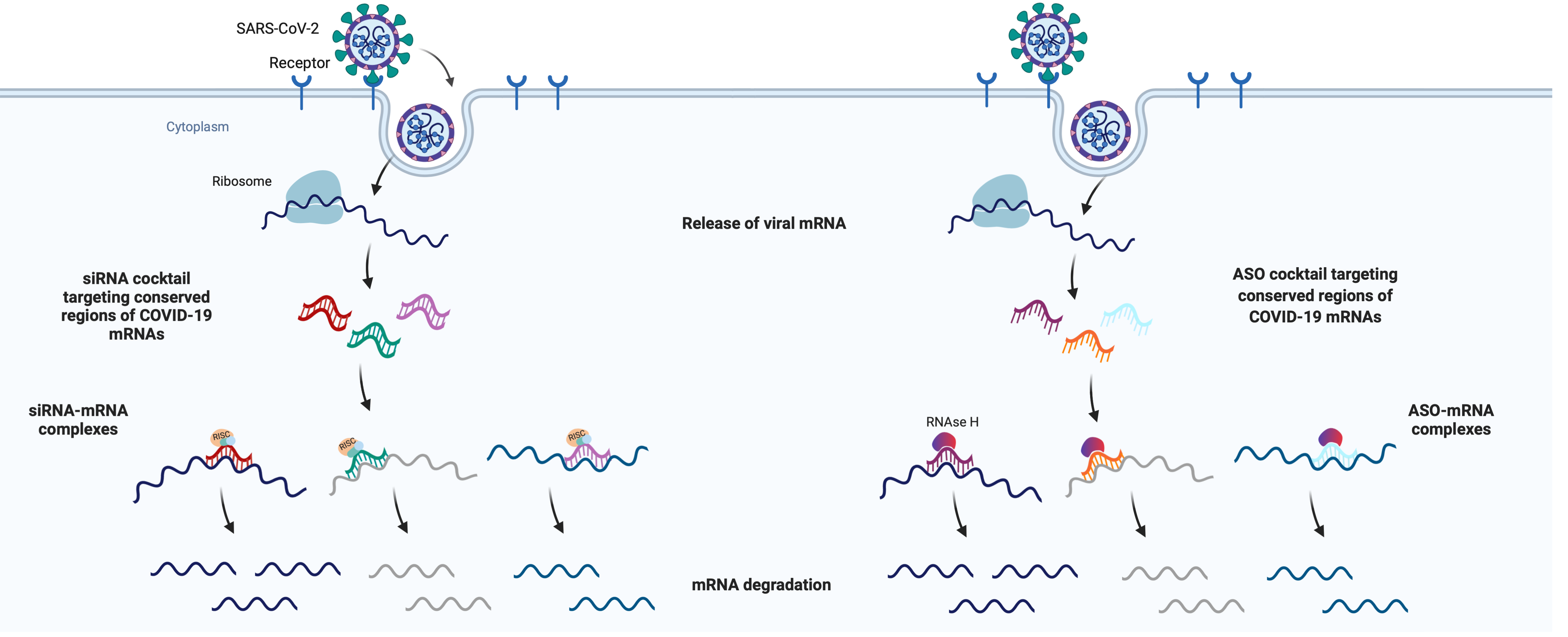
COVID-19 vaccine development
This is your opportunity to take an active role in the fight against coronavirus.
Every dollar you donate goes directly to UMass Chan labs working on front-line research for treatments, tests and vaccines. Please ... won't you join us?