What is a Clinical Trial

What is a Clinical Trial?
Clinical trials are purely voluntary research studies (requiring informed consent - http://www.fda.gov/ForPatients/ClinicalTrials/InformedConsent/ucm20041763.htm) on humans designed by scientists and medical experts to answer questions about a disease and potential new therapies. Clinical trials answer specific questions about safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments. They are an essential and necessary component of the scientific research process. Simply put, there is no other way for research to show that a proposed treatment works.It is important to remember that while the FDA does not conduct Clinical Trials it regulates and approves them. Learn more about clinical trials and find a trial that might be right for you.
How is a Clinical Trial conducted?
There are two type of clinical trials. An Interventional Trial tests whether an experimental treatment or a new way of using an existing therapy is effective and safe. An Observational Tiral studies health issues in a large group of people in their natural environment to determine if certain behavior can increase or decrease the chance of a disease.
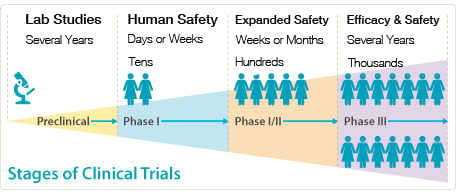
Interventional Clinical trials are conducted in a series of carefully monitored phases designed to answer the following questions.
Phase I: A Phase I trial tests a new drug or treatment for the first time in humans. Therefore, only a small group of people, typically in the tens, are monitored to evaluate the treatment's safety, determine a dosage range, and identify side effects.
Phase II: A Phase II trial studies the effectiveness of the treatment in a larger number of humans, typically in the hundreds.
Phase III: The Phase III trial tests the treatment in a large group of several thousands of people. This large-scale testing gives more detailed information about the drug's benefits, effectiveness, and possible side effects.
The numbers presented here do generally not apply to Clinical Trials in Gene Therapy as genetic diseases are rare, thus in such cases the numbers may be in the tens.
Drugs or treatments that have already been approved by the Federal Food and Drug Administration (FDA) and are believed to be beneficial for other kind of diseases for which the drug was not initially approved for are tested in what is referred to as a Phase IV clinical trial.
Benefits
Participating in a clinical trial can be quite a rewarding experience but it is not without risks. The following benefits and risks should be carefully considered before deciding if you should enroll in a clinical trial.
- You will have access to cutting-edge new treatments and high standards of care from leading professionals
- The treatment may be effective and is provided at no cost.
- Joining a clinical trial will increase your knowledge and understanding of your disease.
- People who take part in clinical trials are contributing to science that may benefit themselves and others. The medications that you take now are available only because people before you have volunteered in clinical trials.
Generally, every effort is made to ensure that a clinical trial is as safe as possible. This begins with selecting the appropriate patients for a clinical tiral. Not every patient that has a particular disease is a good candidate for a clinical trials. There may be many different reasons why you do not qualify for a clinical trial and minimizing the risk for the patient is the prime concern. Nonetheless, clinical trials are not risk free as they test new therapies. Here are some of the risks to consider.
Risks
- There may be undesirable yet unknown side effects to the treatment.
- The treatment may not be effective for the participant.
- The participant is part of the control group receiving a "placebo".
- Some Studies may be quite demanding requiring a lot of travel time.
The Control group is an important part of any clinical trial and used as a basis for comparison to determine the effectiveness of a treatment. In many studies, the researchers themselves are not aware of who is taking the treatment and who is part of the control group to avoid any bias.
Learn more about our clinical trials at UMass
