| Diagnostic Molecular Oncology
Scientific Director: Lloyd Hutchinson, Ph.D. |
|
About Us
Solid Tumors
Hematolymphoid Tumors
Lab Personnel
Faculty
Specimen Collection and Handling
Skin Biopsy for Clonality Analysis (Gene Rearrangement)
Test Directory and Specimen Requirements
Requisition
Contact Information
About Us
The Laboratory of Diagnostic Molecular Oncology provides a wide range of molecular testing. Our testing involves both the diagnosis and monitoring of hematolymphoid malignancies and solid tumors. This testing is performed on a variety of specimen types including blood, bone marrow, fresh or frozen tissue, paraffin embedded tissue and body fluids.
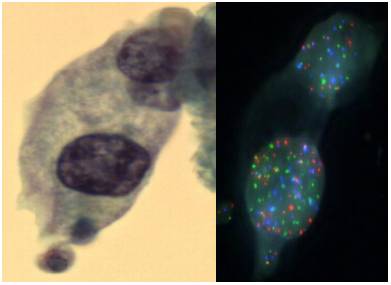
The laboratory occupies a unique niche when it comes to the war on cancer. As a clinical laboratory we get feedback from physicians and patients by helping with early cancer diagnosis and by guiding the most effective patient therapy. As a research laboratory we continue to discover and develop new cancer diagnostics that will support patient care. For example we have developed a novel assay integrating cell morphology with fluorescent in situ hybridization to improve the accuracy of bladder cancer diagnosis (see figure above).
Due to continuing efforts to integrate these novel assays into routine laboratory testing the number of assays performed in-house continues to expand. In addition, this laboratory also coordinates the send-outs of esoteric molecular oncology testing to reference laboratories.
| Solid Tumors |
|
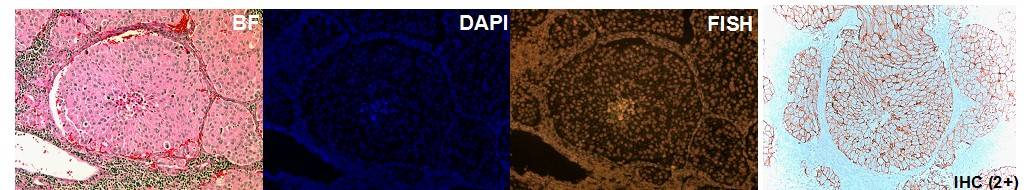
The laboratory of Diagnostic Molecular Oncology performs testing on a variety of solid tumor samples. Testing methods include in situ hybridization (FISH), PCR, and hybrid capture techniques which employ a combination of traditional bench methods and state of the art robotics. We are able to perform testing on fresh and frozen tissue samples as well as formalin fixed paraffin embedded tissue. See the Test Directory for specific details about specimen requirements.
Testing that is performed includes:
- Her2/neu Gene 17p11.1 Amplification
- nMyc Gene 2p24.1 Amplification
1p19q Chromosome Deletion(1p36, 19q13) - UroVysion FISH
- Human Papilloma Virus
Research projects include:
1. Hemizygous Loss of 9p21 in Urine Cytology, as detected by UroVysion Fluorescence in situ hybridization (FISH), is associated with Low Grade Urothelial Carcinomas.
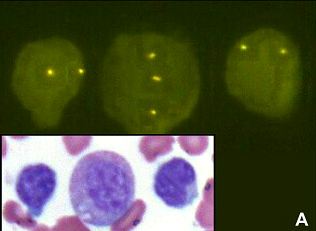
2. Impact of Original FDA and New ASCO/CAP Guidelines for HER2/neu Evaluation in Breast Cancer: IHC and FISH scores using Manual or Quantitative-Automated Imaging Systems and Different Fixatives (see Figure above & below).
 |
The laboratory of Diagnostic Molecular Oncology performs diagnostic testing and minimal residual disease analysis for a variety of hematolymphoid tumors. Testing methods include southern blotting, real time quantitative PCR, capillary gel electrophoresis and Luminex microarray analysis. We perform testing on blood, bone marrow, fresh and frozen tissue samples as well as formalin fixed paraffin embedded tissues. We also accept slides of cut tissue sections. See the Test Directory for specific details about specimen requirements.
Testing that is performed includes:
- T Cell Clonality
- B Cell Clonality
- BCL-2 PCR t(14;18)(q32;q21)
- BCR/ABL Quantitative PCR
- PML/RARA PCR
- Acute Leukemia Multiplex PCR Translocation Panel
- EBV RNA ISH (EBER-1)
Research projects include:
1. BARCODE-ALL: accelerated and cost-effective genetic risk stratification in acute leukemia using spectrally addressable liquid bead microarrays. Leukemia (2003) 17, 1404-1410.
LINKS:
http://www.nature.com/leu/journal/v17/n7/full/2402985a.html
http://www.asuragen.com/diagnostics_home.html
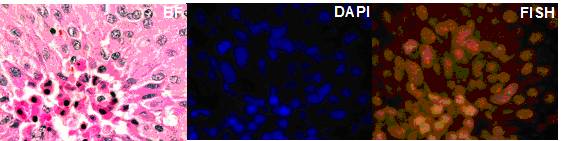
2. Trisomy 8 in B-cell chronic lymphocytic Leukemia. Cancer Genetics and Cytogenetics 183 (2008) 49-52. (Trisomy 8 restricted to myeloid and not lymophcytic lineage, see Figure. above)
Lab Personnel

Faculty
Dr. Bruce Woda, MD
Dr. Lloyd Hutchinson, PhD
Specimen Collection and Handling
Hours of operation are 8:00am to 5:00pm Monday through Friday
- All specimens must be accompanied by a Diagnostic Molecular Oncology Requisition that includes the patient name, medical record number, source of specimen and the submitting physician’s name.
- All samples being submitted to the laboratory must be properly identified by (a.) indicating the patient’s full name, (b) patient’s unique identification number (Medical Record Number or Date of Birth), (c) date and time collected, and (d) the initials of the person collecting the sample. Complete the requisition and place the specimen and requisition from each patient in individual biohazard labeled transport bags.
- An adequate clinical history including pertinent lab/path findings is essential. Failure to provide this information may result in a delay in reporting results.
- Verbal Orders: The Clinical Laboratory Improvement Act of 1988 (CLIA ’88) requires that all verbal requests for laboratory testing MUST BE followed up with written verification of each request. An Add On Test request form can be faxed to you in receipt of any verbal orders.
- All samples (Except fresh skins) are sent to be accessioned at One Biotech. There samples will be registered and received and then triaged to the appropriate departments.
-
Fresh skins are to be collected and shipped with 1-2 hours of collection. See Skin Biopsy for Clonality Analysis directions below.
Skin Biopsy for Clonality Analysis (Gene Rearrangement)
- Place ½ fresh skin biopsy on gauze moistened with saline and put in a specimen container labeled with patient name, date of birth or unit number and specimen date. Place other ½ of biopsy in formalin for Pathologist to do morphologic diagnosis.
- Place containers in a biohazard bag provided and seal. Fill out requisitions and place specimens and requisitions in envelope provided (see picture).
- Deliver the specimens directly to Three Biotech, Innovation Drive, 2nd floor, Molecular Pathology, Room 276, Attention: Lloyd Hutchinson, Ph.D. DO NOT SEND THE SPECIMEN TO CENTRAL PROCESSING (One Biotech). THIS WILL CAUSE UNNECESSARY DELAY.
- Call the laboratory at 508-793-6240 to let us know the specimen is coming. If you are not able to speak with a technician, page us at 508-899-6500 and enter your call back number after the beep.
- For pick-up of the specimen within the Memorial-University-Hahnemann-Biotech1-Biotech3 loop, call extension 4-5120 for the Stat courier service.
- For pick-up of the specimen outside the UMASS Memorial-University-Hahnemann-Biotech1-Biotech3 loop call Customer Service at 508-334-2863 and request a stat pick up.
- If the specimens cannot be shipped in time to arrive during operational laboratory hours (8 AM to 5 PM, Monday – Friday), freeze the fresh skin specimen and ship the formalin-fixed specimen using standard procedures. When normal business hours resume, ship the fresh skin specimen at room-temperature using the STAT courier.
For additional STAT labels and envelopes call the Laboratory of Diagnostic Molecular Onocology at 508-793-6240 or print the labels at the end of this document.
|
STAT SPECIMENFORWARD IMMEDIATELY
SEND TO: Biotech 3, 2nd floor Molecular Pathology Lab –Rm 276 Attn: Lloyd Hutchinson
Call (508) 793-6240
|
|
STAT SPECIMENFORWARD IMMEDIATELY
SEND TO: Biotech 3, 2nd floor Molecular Pathology Lab –Rm 276 Attn: Lloyd Hutchinson
Call (508) 793-6240
|
Test Directory and Specimen Requirements
| B Cell Clonality (Lymphoma) |
|
|
|
|
||
|
Methodology: |
DNA analysis |
|
|
|
|
Performed: |
Mon, Wed, Fri |
|||
|
Reported: |
7-10 days |
|||
|
CPT: |
83907, 83891, 83894, 83912 |
|||
|
|
Specimen Requirements: |
|
Collect: Peripheral blood: Bone Marrow: Fresh tissue:
Fine Needle Aspirate: Block: Slides: |
3 Lavender -top EDTA Tubes 1 Lavender-top EDTA Tube 5x5x5 mm - place tissue on gauze moistened with sterile saline - Notify Lab 508-793-6240 and call STAT courier for pickup. Must be received with 2hr. ThinPrep PreservCyt collection vial
Ten uncharged slides with 10 micron sections |
|
Min. Volume: |
10 mL peripheral blood, 2mL bone marrow |
|
Transport: |
Fresh tissue within 2hr. Blood/Bone marrow at room temp within 24hr or refrigerated within 72 hr. |
|
Notes: |
Test sensitivity is limited to 1% of lymphocyte population |
| T Cell Clonality (Lymphoma) |
|
|
|
|
||
|
Methodology: |
DNA analysis |
|
|
|
|
Performed: |
Tues, Thurs |
|||
|
Reported: |
7-10 days |
|||
|
CPT: |
83907, 83891, 83898, 83903, 83912 |
|||
|
|
Specimen Requirements: |
|
Collect: Peripheral blood: Bone Marrow: Fresh tissue:
Fine Needle Aspirate: Block: Slides: |
3 Lavender -top EDTA Tubes 1 Lavender-top EDTA Tube 5x5x5 mm - place tissue on gauze moistened with sterile saline - Notify Lab 508-793-6240 and call STAT courier for pickup. Must be received with 2hr. ThinPrep PreservCyt collection vial Formalin Fixed Paraffin embedded tissue block Ten uncharged slides with 10 micron sections |
|
Min. Volume: |
10 mL peripheral blood, 2mL bone marrow |
|
Transport: |
Fresh tissue within 2hr. Blood/Bone marrow at room temp within 24hr or refrigerated within 72 hr. |
|
Notes: |
Test sensitivity is limited to 1% of lymphocyte population |
|
|
|
| BCR/ABL QUANTITATIVE PCR, t(9;22)(q34;q11) |
|
|
|
|
|
Methodology: |
RNA analysis |
|
|
Performed: |
Wednesday |
|
|
Reported: |
7-10 days |
|
|
CPT: |
83907, 83891, 83902x3 83898x3, 83896x3, 83912 |
|
|
Specimen Requirements: |
|
Collect: Peripheral blood: Bone Marrow: |
3 Lavender -top EDTA Tubes (preferred specimen type) 1 Lavender-top EDTA Tube |
|
Min. Volume: |
10 mL peripheral blood, 2mL bone marrow |
|
Transport: |
Blood/Bone marrow at room temp within 24hr or refrigerated within 72 hr. |
|
Notes: |
This test is intended for minimal residual disease detection in patients with CML, ALL and AML. Baseline analysis is recommended. |
| BCL-2 PCR, t(14;18)(q32;q21) (Follicular Lymphoma) |
|
|
|
|
|
|
Methodology: |
DNA analysis |
|
|
|
Performed: |
As needed |
||
|
Reported: |
7-10 days |
||
|
CPT: |
83907, 83891, 83898x2, 83896x2, 83894x2, 83912 |
||
|
|
Specimen Requirements: |
|
Collect: Peripheral blood: Bone Marrow: Fresh tissue:
Fine Needle Aspirate: |
3 Lavender -top EDTA Tubes 1 Lavender-top EDTA Tube 5x5x5 mm - place tissue on gauze moistened with sterile saline - Notify Lab 508-793-6240 and call STAT courier for pickup. Must be received with 2hr. ThinPrep PreservCyt collection vial |
|
Min. Volume: |
10 mL peripheral blood, 2mL bone marrow |
|
Transport: |
Fresh tissue within 2hr. Blood/Bone marrow at room temp within 24hr or refrigerated within 72 hr. |
|
Notes: |
This test is intended for minimal residual disease detection. Baseline analysis is recommended. |
|
PML/RARA PCR, t(15;17)(q22;q11.2) |
|
|
|
|
|
Methodology: |
RNA analysis |
|
|
Performed: |
As needed |
|
|
Reported: |
7-10 days |
|
|
CPT: |
83907, 83891, 83902 83898, 83896, 83894, 83912 |
|
|
Specimen Requirements: |
|
Collect: Peripheral blood: Bone Marrow: |
3 Lavender -top EDTA Tubes 1 Lavender-top EDTA Tube |
|
Min. Volume: |
10 mL peripheral blood, 2mL bone marrow |
|
Transport: |
Blood/Bone marrow at room temp within 24hr or refrigerated within 72 hr. |
|
Notes: |
This test is intended for minimal residual disease detection in patients with promyelocytic leukemia (PML). Baseline analysis is recommended. |
| Acute Leukemia Chromosome Translocation Panel |
|
|
|
|
|
Methodology: |
Luminex Microarray |
|
|
Performed: |
As needed |
|
|
Reported: |
7-10 days |
|
|
CPT: |
83907, 83891, 83902, 83900, 83901x4, 83894, 83896x7, 83912 |
|
|
Specimen Requirements: |
|
Collect: Peripheral blood: Bone Marrow: |
3 Lavender -top EDTA Tubes 1 Lavender-top EDTA Tube |
|
Min. Volume: |
10 mL peripheral blood, 2mL bone marrow |
|
Transport: |
Blood/Bone marrow at room temp within 24hr or refrigerated within 72 hr. |
|
Notes: |
This test is intended to identify translocations in acute leukemia (e.g. MLL/AF4 t(4;11), E2A/PBX1 t(1;19), TEL/AML t(12;21), BCR/ABL t(9;22). Call Diagnostic Molecular Oncology Laboratory 508-793-6240 for additional translocation information. |
| EBV RNA ISH (EBER-1) on Tissue |
|
|
|
|
|
Methodology: |
In Situ Hybridization |
|
|
Performed: |
As needed |
|
|
Reported: |
7-10 days |
|
|
CPT: |
88365 |
|
|
Specimen Requirements: |
|
Collect: |
Formalin Fixed Paraffin embedded tissue block |
|
Transport: |
Room temp or refrigerated. |
|
Notes: |
This test is intended for tissue specimens only. |
| HER2/neu Gene 17p11.1 Amplification |
|
|
|
|
|
Methodology: |
Fluorescent In Situ Hybridization |
|
|
Performed: |
Tues, Thurs |
|
|
Reported: |
2-4 days |
|
|
CPT: |
88368x2 |
|
|
Specimen Requirements: |
|
Collect: Block: Slides: |
Formalin Fixed Paraffin embedded tissue block 3 charged slides with 5 micron sections and 1 H&E slide |
|
Transport: |
Room temp or refrigerated. |
|
Notes: |
This test is intended for patients with invasive breast cancer. |
|
nMyc Gene 2p24.1 Amplification |
|
|
|
|
|
Methodology: |
Fluorescent In Situ Hybridization |
|
|
Performed: |
Tues, Thurs |
|
|
Reported: |
2-4 days |
|
|
CPT: |
88368x2 |
|
|
Specimen Requirements: |
|
Collect: Block: Slides: |
Formalin Fixed Paraffin embedded tissue block 3 charged slides with 5 micron sections and 1 H&E slide |
|
Transport: |
Room temp or refrigerated. |
|
Notes: |
This test is intended for patients with Neuroblastoma, Retinoblastoma, small cell lung carcinoma. |
|
1p19q Chromosome Deletion (1p36, 19q13) |
|
|
|
|
|
Methodology: |
Fluorescent In Situ Hybridization |
|
|
Performed: |
Tues, Thurs |
|
|
Reported: |
2-4 days |
|
|
CPT: |
88368x2 |
|
|
Specimen Requirements: |
|
Collect: Block: Slides: |
Formalin Fixed Paraffin embedded tissue block 3 charged slides with 5 micron sections and 1 H&E slide |
|
Transport: |
Room temp or refrigerated. |
|
Notes: |
This test is intended for patients with oligodendroglioma |
|
UroVysion Chromosome Aneuploidy |
|
|
|
|
||
|
Methodology: |
Fluorescent In Situ Hybridization |
|
|
|
|
Performed: |
Tues, Thurs |
|||
|
Reported: |
10-14 days |
|||
|
CPT: |
88367x4 |
|||
|
|
Specimen Requirements: |
|
Collect: Voided/Cath. Urine: Bladder/Ureter Wash: Processed Urine/Wash: Cytology Slide: Tissue Block: Tissue Slides: |
50mL with preservative
PAP stained ThinPrep or UroCyte slide Formalin Fixed Paraffin embedded tissue block 3 charged slides with 5 micron sections and 1 H&E slide |
|
Collection Notes: |
Call the Cytology laboratory for preservative (508-793-6136) |
|
Min. Volume: |
Urine/Wash 50mL |
|
Transport: |
Room temp. Urine/Wash without preservative within 24hr, with preservative within 72hr. |
|
Notes: |
This test is intended for patients with hematuria or a history of bladder cancer. |
| HPV – High Risk |
|
|
|
|
|
Methodology: |
Hybrid Capture |
|
|
Performed: |
Daily |
|
|
Reported: |
2 days |
|
|
CPT: |
87621 |
|
|
Specimen Requirements: |
|
Collect: Cytology PAP Test: Biopsy Material: Fresh Biopsy:
|
ThinPrep PreservCyt collection vial ThinPrep PreservCyt collection vial 5x5x5 mm - place tissue on gauze moistened with sterile saline - Notify Lab 508-793-6240 and call STAT courier for pickup. Must be received with 2hr. |
|
Collection Notes: |
This test is not intended for paraffin embedded tissue. |
|
Min. Volume: |
4 mL cervical specimen. Residual fluid after PAP test is sufficient. See Cytology PAP test for additional instructions. |
|
Transport: |
Room temp. Stability 3 months |
Contact Information
Laboratory of Diagnostic Molecular Oncology
UMass Memorial Medical Center
Department of Pathology
1 Innovation Drive
Three Biotech, Room 276
Worcester, Ma 01605
PH 508-793-6240
PH 508-793-6244
Fax 508-793-6242