New article in Molecular Therapy
Dr Mueller and colleagues published a new research article in Molecular Therapy, in collaboration with Dr Flotte from UMass, entitled "5 Year Expression and Neutrophil Defect Repair After Gene Therapy in Alpha-1 Antitrypsin Deficiency".
Abstract: Alpha-1 antitrypsin deficiency is a monogenic disorder resulting in emphysema due principally to the unopposed effects of neutrophil elastase. We previously reported achieving plasma wildtype alpha-1 antitrypsin concentrations at 2.5-3.8% of the purported therapeutic level at 1 year after a single intramuscular administration of recombinant adeno-associated virus serotype 1 alpha-1 antitrypsin vector in alpha-1 antitrypsin deficient patients. We analyzed blood and muscle for alpha-1 antitrypsin expression and immune cell response. We also assayed previously reported markers of neutrophil function known to be altered in alpha-1 antitrypsin deficient patients. Here, we report sustained expression at 2.0-2.5% of the target level from years 1-5 in these same patients without any additional recombinant adeno-associated virus serotype-1 alpha-1 antitrypsin vector administration. In addition, we observed partial correction of diseaseassociated neutrophil defects, including neutrophil elastase inhibition, markers of degranulation and membrane-bound anti-neutrophil antibodies. There was also evidence of an active T regulatory cell response (similar to the 1 year data) and an exhausted cytotoxic T-cell response to adeno-associated virus serotype-1 capsid. These findings suggest that muscle-based alpha-1 antitrypsin gene replacement is tolerogenic and that stable levels of M-AAT may exert beneficial neutrophil effects at lower concentrations than previously anticipated.
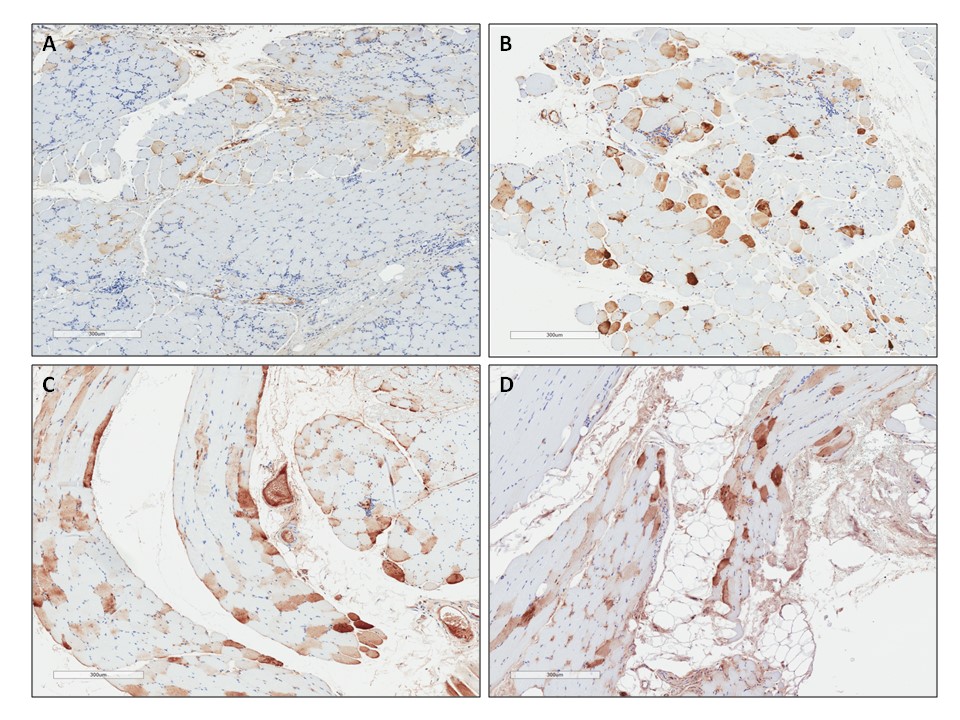
Figure 2. M-AAT expression in skeletal muscle 3 months, 12 months and 5 years after intramuscular administration of rAAV1-CB-hAAT. Muscle immunochemistry staining for hAAT. Specimens were biopsied from the area injected with rAAV1and the tissue stained for hAAT (brown). AAT expression was persistent whileinflammatory cell infiltrates decreased over time from 3 months to 5 years. All slides are from Patient 308. A) Tissue 3 months after rAAV1-CB-hAAT delivery. B) Tissue 12 months after rAAV1-CB-hAAT delivery. C) and D) Tissue 5 years after rAAV1-CB-hAAT delivery.
The epub is accessible here.