Strengthening Translational Research in Diverse Enrollment (STRIDE)
Strengthening Translational Research in Diverse Enrollment (STRIDE) is a partnership of the Clinical and Translational Science Awards (CTSAs) at the UMass Chan Medical School (UMass Chan), the University of Alabama at Birmingham (UAB), and Vanderbilt University School of Medicine along with the Community Campus Partnerships for Health (CCPH), an international leader in community-engaged research. The STRIDE project aims to create tools that can be shared with researchers to help them recruit and deliver informed consent that is culturally and literacy appropriate. On this page dedicated to STRIDE course participants, what would you like to access?






Course


STRIDE Is a Partnership
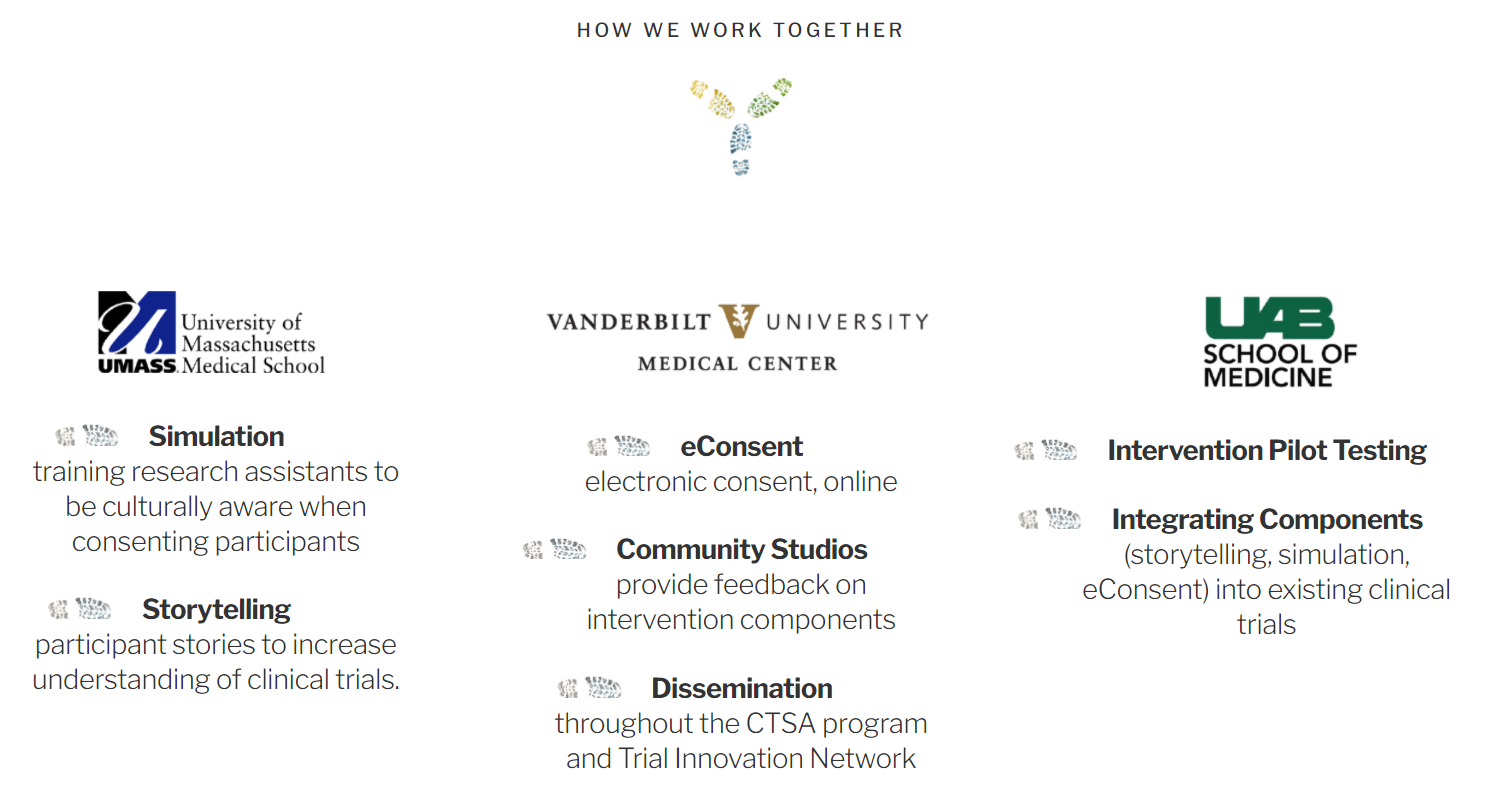
In this partnership, UMass Chan Medical School provides the Simulation element, with a goal of training research assistants to be culturally aware when consenting participants; as well as the Storytelling element, with participant stories to increase understanding of clinical trials.
The Vanderbilt University School of Medicine provides the eConsent element, for electronic consent, online; the Community Studios, where participants provide feedback on intervention components; and the Dissemination element, throughout the CTSA program and Trial Innovation Network.
The University of Alabama at Birmingham (UAB) provides Intervention Pilot Testing; and Integrating Components of storytelling, simulation, and eContent into existing clinical trials.
The Element of Simulation in STRIDE
The research team component of the STRIDE intervention hinges on an innovative application of medical simulation to improve the cultural sensitivity of those recruiting and enrolling diverse participants.
Through this process, trained community members served as “Acting Research Participants” similar to the well-established standardized-patients methodology. Acting Research Participants engage with research assistants in mock recruitment scenarios for enrollment in a clinical trial in the setting of the simulation center, which is capable of replicating common recruitment venues and providing hands-on, experiential learning in a structured environment.
Practice in this safe environment promotes learning critical communication skills to address emotionally sensitive, cross-cultural issues that arise in the process of informed consent. This process was developed through a bi-directional partnership with our community partners.
The Element of Storytelling in STRIDE
“Storytelling is a proven behavioral intervention where people in a community tell their health stories to others to improve health outcomes.”
Process
- Interview guides were developed based on the thematic analysis developed in focus groups that occurred at the University of Alabama-Birmingham.
- Vanderbilt selected individuals to participate in one-on-one on camera interviews, and these interviews were conducted by the UMass Storytelling Team.
- The Storytelling team conducted the video-recorded in depth interviews based on the alignment of the themes developed and the content of their story. We coordinated the interviews to be convenient to the individual participants. We staggered the video interviews so that we can adapt our process as necessary. During the interviews, each participant was reminded to describe their best stories.
- The videos were subsequently divided into “story units”, or themes (reason for participation, informed consent, etc.
- Each story unit will be systematically rated and reviewed by members of the UMass team. The storytellers will also be rated by community members and other outside evaluators in order to pick the best “stories” that will be packaged into the final intervention.
Learn about the role of Storytelling in STRIDE in these 4 videos.
Why should I participate?
Video length: 2 minutes 30 seconds
What should I know before participating?
Video length: 1 minute 30 seconds
Is it safe to participate?
Video length: 1 minute 30 seconds
Who should be participating in Research?
Video length: 2 minutes
The Use of eConsent
Electronic Consent (eConsent) is a new way of helping patients consent for a research study online. Patients can sign an eConsent document on site or at home using a computer or tablet. eConsent may also use to help patients better understand what to expect from the study.
The STRIDE intervention uses adapts eConsent, based in the REDCap platform, to be culturally sensitive to African American and Hispanic communities. REDCap is a secure, web application that lets researchers build and manage surveys and databases. REDCap meets standards for patient information security (HIPPA) and patients can access REDCap eConsent forms on a computer, smartphone, or tablet.
By moving consent online, we have the chance to include things like pictures, audio, and avatars to help participants feel more comfortable and have a better understanding of the risks and benefits of a study.
Use of eConsent also lets participants review information at their own pace, which may help participants to feel less anxious and less pressured to participate.
The Role of Community Investigators
Community involvement and input is a key part of the STRIDE project. Each of the 3 STRIDE sites identified 1-2 Community Members who would provide feedback on all parts of the project. In addition, Community Investigators from each site meet monthly. Community Investigators will also be trained to evaluate standardize patient encounters and will help to train research assistants in the studies partnered with STRIDE.
Start by Taking the Implicit Association Test (IAT)
The Implicit Association Test (IAT) measures attitudes and beliefs that people may be unwilling or unable to report. It may be especially interesting if it shows that you have an implicit attitude that you did not know about. IAT does this through a test that measures the strength of associations between concepts (e.g., black people, gay people) and evaluations (e.g., good, bad) or stereotypes (e.g., athletic, clumsy).
The IAT is brought to you by Project Implicit, founded in 1998 by three scientists – Dr. Tony Greenwald (University of Washington), Dr. Mahzarin Banaji (Harvard University), and Dr. Brian Nosek (University of Virginia). Project Implicit Health (formerly Project Implicit Mental Health) launched in 2011 and is led by Dr. Bethany Teachman (University of Virginia) and Dr. Matt Nock (Harvard University). The mission of Project Implicit is to educate the public about bias and to provide a “virtual laboratory” for collecting data on the internet. Project Implicit scientists produce high-impact research that forms the basis of our scientific knowledge about bias and disparities.
In the video below, we learn about the IAT and the important data it has generated. Video length: 5 minutes
This video is provided by BruinX, the Research and Development unit within University of California, Los Angeles (UCLA)'s Office of Equity, Diversity and Inclusion.
Click here to take the Implicit Association Test (IAT)
Half-Day STRIDE Workshop
Thank you for your interest in the Half-Day Workshop. The goals for this workshop include increasing equitable communication and cultural humility through simulation and deliberate practice. This requires considering our own attitudes, ideas and feelings about others and depends on our creating a supportive environment for that important work to take place. Please review the information below and reach out if you have any questions. We look forward to working with you!
Workshop participants: Login to view materials
Testimonials
“It's always helpful to be more mindful of our own biases and this workshop helped remind us that when we feel a bias, when that signal fires in our brain, to stop and think about what/where that is coming from.”
“This was extremely thought provocative. It helped me to do some reassessment of myself.”
“Lovely workshop. Wonderful experience. #Supportive. #Positive Change.”
“Please find a way to spread this work far and wide."
Sample Schedule for an afternoon/evening Half-day STRIDE Workshop
|
Time |
Minutes |
Agenda Topic |
|
3:00 pm |
5 |
Welcome, Introductions, Overview |
|
3:05 pm |
35 |
Self-assessment including pretest with video reflection |
|
3:40 pm |
15 |
Bias and how it impacts teams and patient interactions |
|
3:55 pm |
60 |
Implicit bias |
|
4:55 pm |
10 |
Break |
|
5:05 pm |
30 |
Equity-based communication and cultural humility |
|
5:35 pm |
75 |
Group Standardized Patient (SP) interaction and deliberate practice - 2 rounds
|
|
6:50 pm |
10 |
Larger group debrief |
|
7:00 pm |
5 |
Post-test and program evaluation |
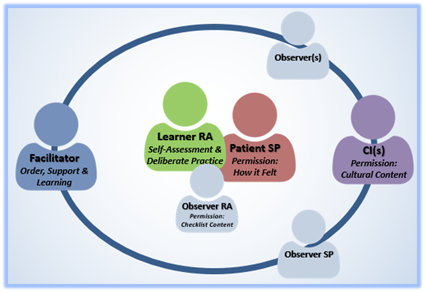
The Fishbowl Method
Fishbowl Debriefing helps learners visualize the entire process, with themselves at the center. It also reinforces roles, especially the role of the facilitator in directing the discussion toward limited/prioritized feedback and creating a supportive space for learners to practice skills.
Two-Day STRIDE Workshop
Thank you for your interest in the Two-Day Workshop. The goals for this workshop include increasing equitable communication and cultural humility through simulation and deliberate practice. This requires considering our own attitudes, ideas and feelings about others and depends on our creating a supportive environment for that important work to take place. Please review the information below and reach out if you have any questions. We look forward to working with you!
About STRIDE
STRIDE (Strengthening Translational Research in Diverse Enrollment) is a multi-institutional NIH-funded initiative which combines econsent, storytelling, pilot testing and simulation with the goal of increasing diversity of enrollment in clinical research. STRIDE engaged community investigators (CIs) in the design and implementation of a simulation-based workshop to build RA capacity to recognize and respond to unconscious bias during informed consent interactions and enhance respectful, culturally informed research participant engagement. Read more
Planning Documents:
Program Slides:
-
Part 1 - Introduction and Learning to Sim
-
Part 2 - Understanding Implicit Bias
-
Part 3 - Sims 2, 3, 4 & the Fishbowl
Mock Cases:
-
Research Assistant Pre and Post-Intervention Questionnaire
-
Diversity Petal
-
Potential Research Participant Descriptions
-
Checklist
-
Rating Scale Guide
-
Commitments to Change
Customized Workshop through Individual Consultation
Explore the opportunity for a customized workshop through individual consultation with us!
Contact Us Now:
Let Us Help
Build Your Own Simulation Workshop
Last Updated Aug 1st, 2021
You might also be interested in:



