Research
How Can We Enable And Enhance AAV Gene Therapy?
Our work focuses on understanding adeno associated viruses (AAV) to deliver gene therapy and the host response. A critical component of AAV gene therapy is the immune response to both the method of delivery, and the genetic cargo that is being delivered. Using chimeric antigen receptor technology (CAR) we have developed AAV specific CAR T cells to mimic immune responses in some trials to AAV gene therapy and powerful CAR-T regulator cells (CAR Tregs) which enable suppress an immune response and enable AAV gene therapy.
The Problem Of Differing Immune Responses During Clinical Trials Against AAV Gene Therapy
The immune responses against AAV (the method of gene delivery) and delivered transgenes (the genetic cargo) have limited the therapeutic potential of AAV gene therapy.
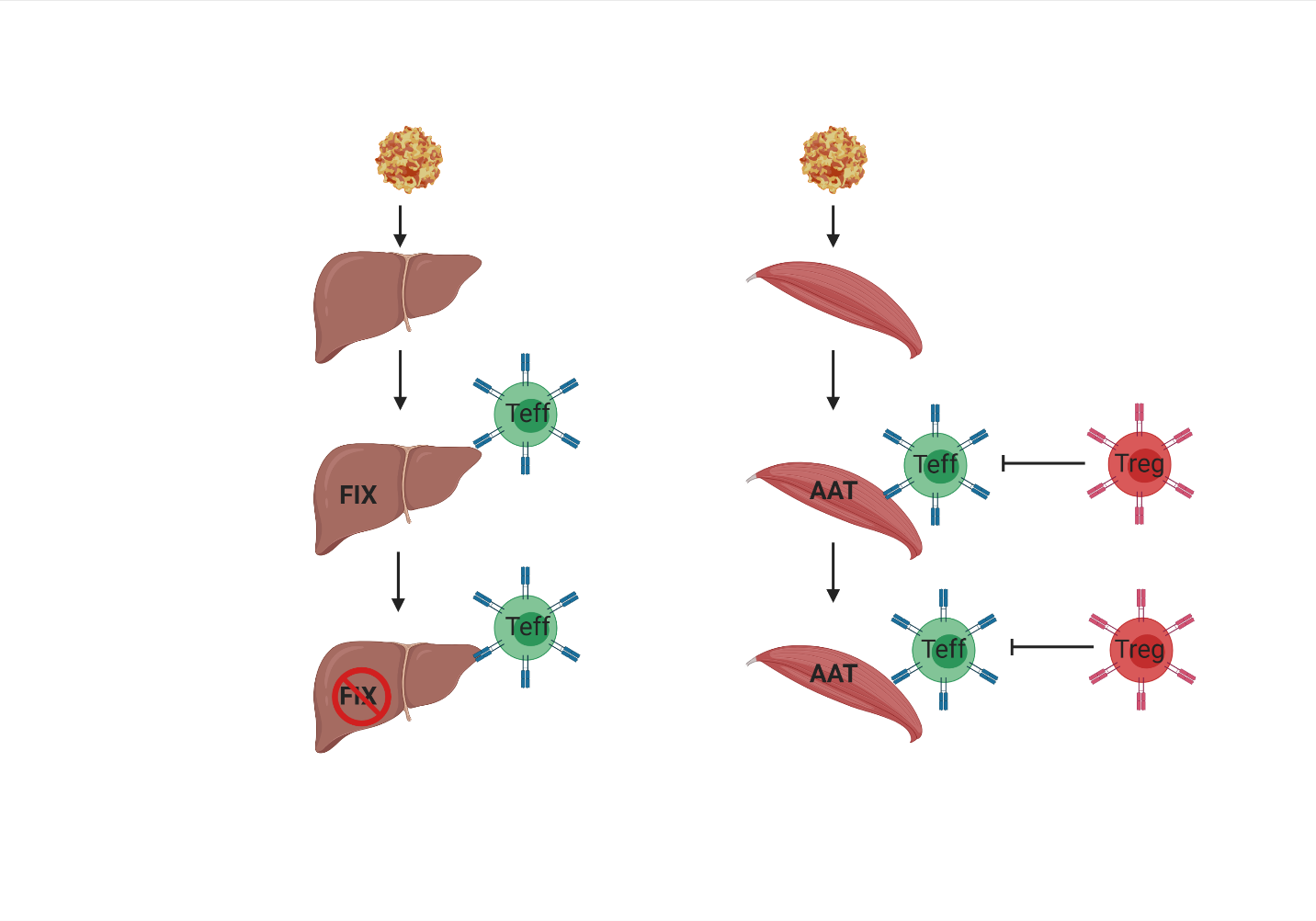
In some clinical trials, effector T cell responses to AAV capsid have been observed to limit transgene expression in clinical trials of IV rAAV gene therapy, such as in hemophilia, a type of immune response that was not accurately predicted by animal models.
Conversely, in other clinical trials, with intramuscular delivery, with and without immune suppression, also reported significant immune infiltration, but minimal clearance of transgene expression. This was attributed to an immune modulatory role of T-regulatory cells identified within the injected muscle.
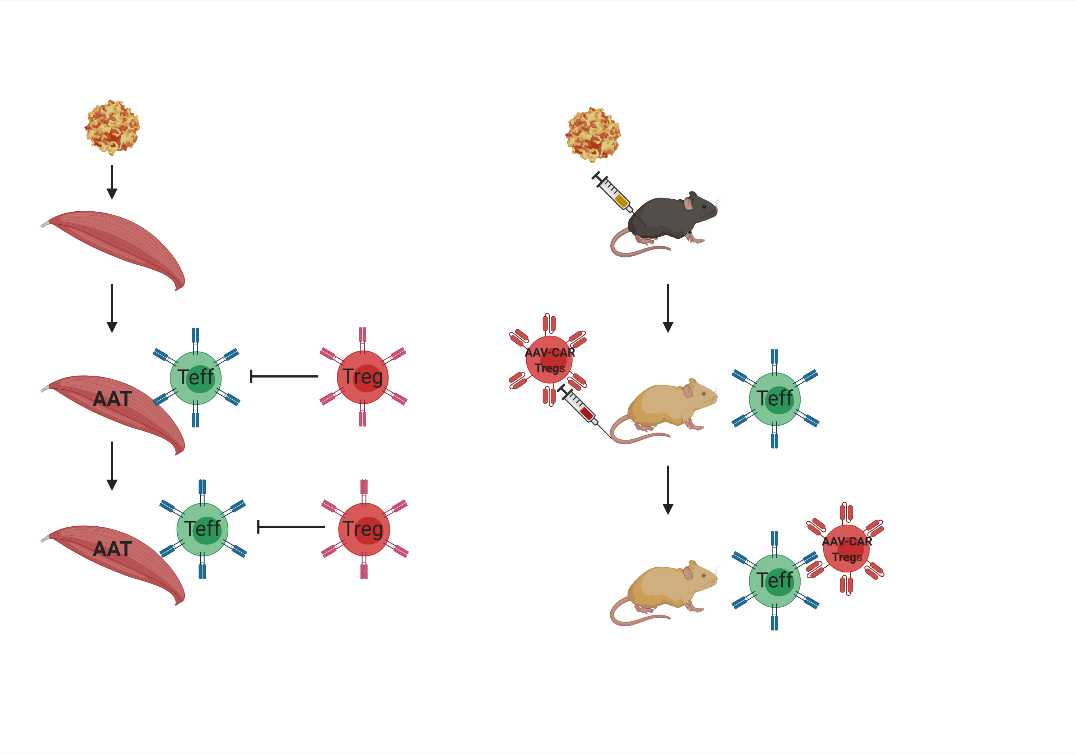
Creating AAV specific CAR T cells to model capsid specific immune response
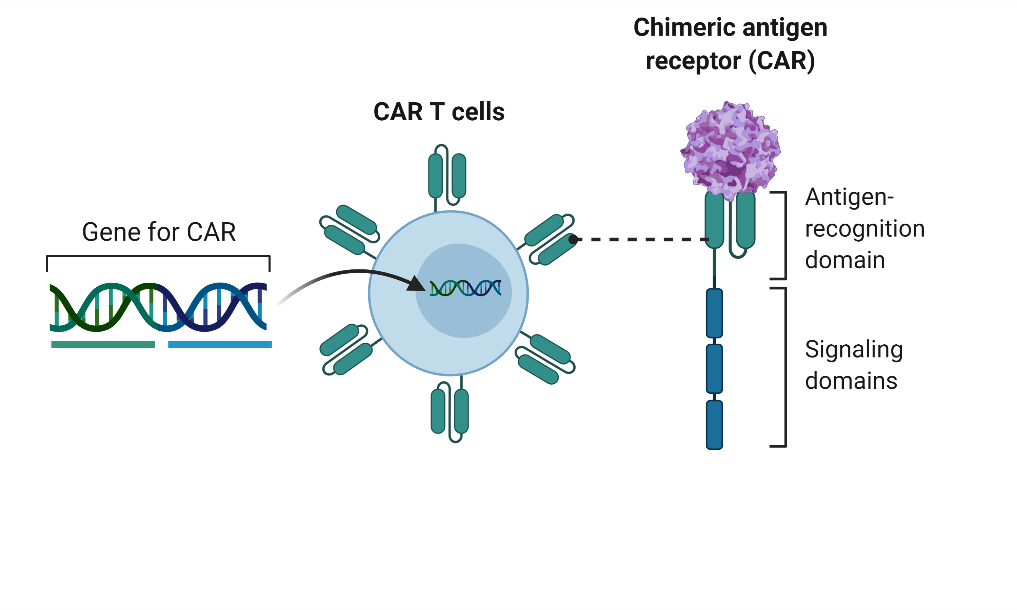
The differing responses to AAV were not observed or predicted in preclinical animal studies. To better understand the capsid immune response, we have created T-cells specific for AAV capsid using chimeric antigen receptor (CAR) technology. We created CAR T cells specific for AAV capsid using an scFv from a previously published anti-AAV antibody, and a 3rd generation CAR construct.
 To model the clinical cellular immune response against AAV capsid, we injected AAV-CAR T cells into AAV-injected animals which resulted in the clearance of transgene expression.
To model the clinical cellular immune response against AAV capsid, we injected AAV-CAR T cells into AAV-injected animals which resulted in the clearance of transgene expression.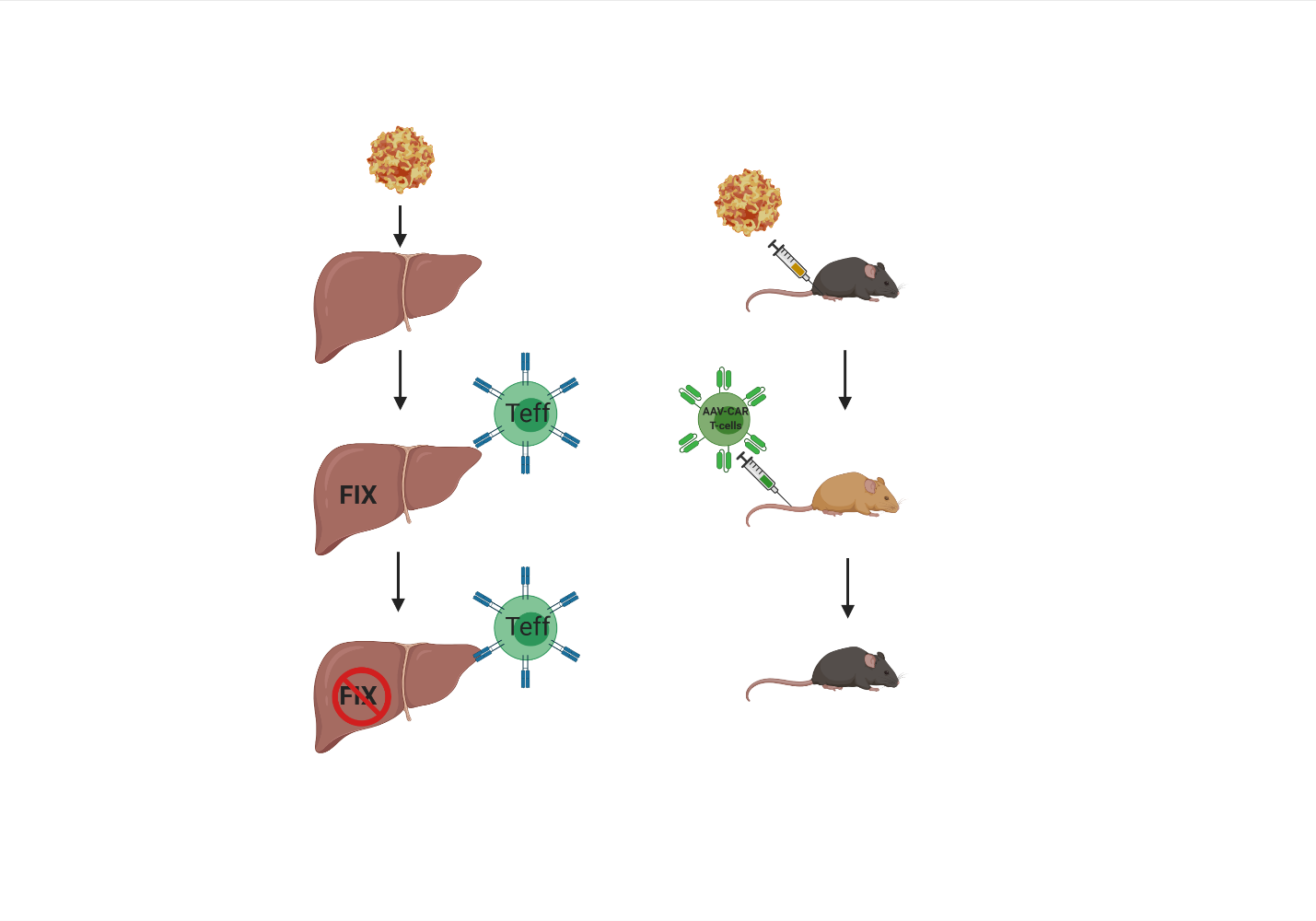
Mitigating the immune response against AAV and immunogenic transgene using AAV-CAR Tregs
Now that we established a model to mimic what was observed in humans, we developed ways to enhance and enable AAV gene therapy by suppressing the host response. We focused on the critical observation that during intramuscular delivery of AAV, it resulted in the infiltration of effector T cells along with regulatory T cells and the persistence of transgene expression.
We hypothesized that the regulatory T cells are allowing for sustained transgene expression. To create T-regulatory cells we added a 2A sequence to the CAR construct and a FoxP3. FoxP3 is a master transcription factor in regulatory T cells and several studies showed that the expression of FoxP3 in T cells creates stable and functional regulatory T cells.
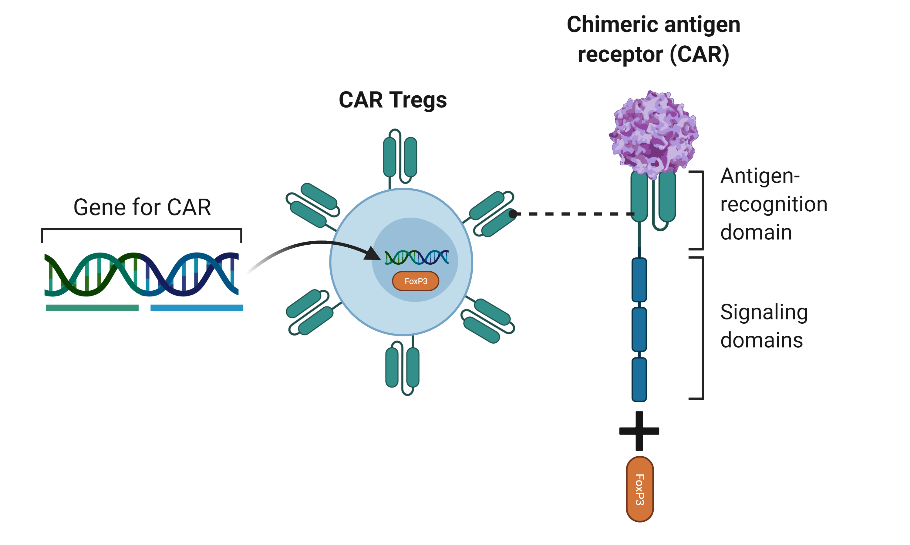
To examine the immune modulatory ability of AAV-CAR Tregs in-vivo against immunogenic capsid and transgene, we employed the previously described immune response against AAV-rh32.33 capsid and ovalbumin respectively. The delivery of AAV-CAR Tregs resulted in an enhancement in transgene expression in both experiments and production of immune suppressive cytokines.
Future Directions
We are now focusing on the ability of AAV-CAR Tregs to suppress the preexisting immune responses against both the AAV capsid and the introduced transgene. Our goal is to enable the effective use of AAV gene therapy with AAV-CAR Tregs. We are also investigating AAV-CAR Tregs ability to treat autoimmune and neuroinflammatory diseases and are excited to collaborate on this promising work!
