Glibenclamide for Large Hemispheric Infarction Analyzing mRS and Mortality

Site Primary Investigator (UMASS): Raphael A. Carandang, MD.
Study Coordinator: Lauren Vilchinsky, lauren.vilchinsky@umassmed.edu, 508-856-4667
Design: A randomized, multi-center, placebo-controlled, double-blinded trial.
Funded by: Biogen Inc.
Objectives:
Primary Objective:
-To determine if BIIB093 improves functional outcome in comparison to placebo in patients with large hemispheric ischemic stroke.
Secondary Objectives:
-To determine BIIB093’s safety profile, improvement in survival, and reduction in midline shift in comparison to placebo use.
Outcome measures: Improvement of mRS at day 90.
Anticipated study duration: 4-5 years
Anticipated patient enrollment: Total of 600 subjects and up to 80 subjects with age >70 years and up to 85 years (inclusive).
Clinicaltrials.gov Link: https://clinicaltrials.gov/ct2/show/NCT02864953?term=BIIB093&rank=1
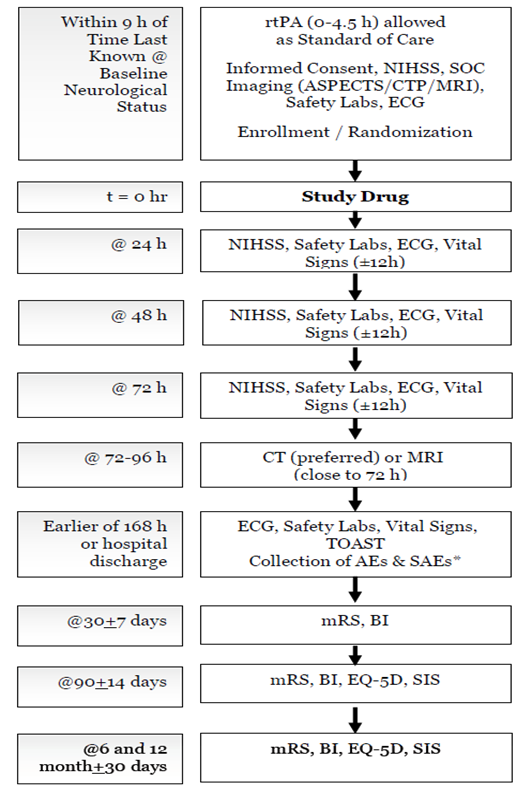
AE = adverse event; ASPECTS = Alberta Stroke Program Early CT Score; BI = Barthel index; CT = computerized tomography; CTP = computed tomography perfusion; ECG =electrocardiogram; EQ-5D = EuroQol; MRI = magnetic resonance imaging; mRS = modified Rankin scale; NIHSS = National Institues of Health Stroke Scale; rtPA = recombinant tissue plasminogen activator; SAE = serious adverse event; SIS = stroke impact scale; SOC =standard of care; TOAST = trial of Org 10172 in acute stroke treatment
*Cirara is intravenoues Glyburide
IND number: 128581; EnduraCT number: 2016-001932-36
