Monday, December 30, 2019
|
I previously wrote a blog to give the details of a Phase II, placebo-controlled, randomized clinical trial to test topical ruxolitinib cream as a treatment for vitiligo. The trial was sponsored by the pharmaceutical company Incyte and recruited 157 participants from 26 sites all over the US. You can read the details of the study here, which I won’t repeat again in this post. There I provided the 24-week results, while this post will serve to update everyone on the continued improvement up to 52 weeks, or 1 year.
After 24 weeks, patients who were receiving placebo cream or receiving the lowest dose of drug were re-randomized to a higher dose of the drug – this way everyone who entered the trial got a chance at testing the drug. For this first year, everyone was “blinded”, meaning neither the patients nor the investigators knew who was getting what dose of the drug. After being in the trial for a full year, every patient was given the highest dose of the drug and allowed to use nbUVB as well, if they wanted. Participants are in the second year of this phase right now, and I’ll let you all know how that went when I do!
But for now, here are the results at 1 year
There was substantial improvement over what was seen and reported after only 24 weeks. For those using the highest dose of drug, 58% achieved at least 50% improvement and 51% achieved at least 75% improvement on their face after 1 year – so most patients who got at least 50% better on their face also got over 75% better as well. This is important because most patients want to see at least 75% improvement to feel that their treatment was successful. Only 30% reached 75% improvement at the 6-month mark, so more people achieved this better milestone after a few more months. By 1 year, 33% of patients using the highest dose of drug noted over 90% improvement on their face, which is a great result for those who achieved it.
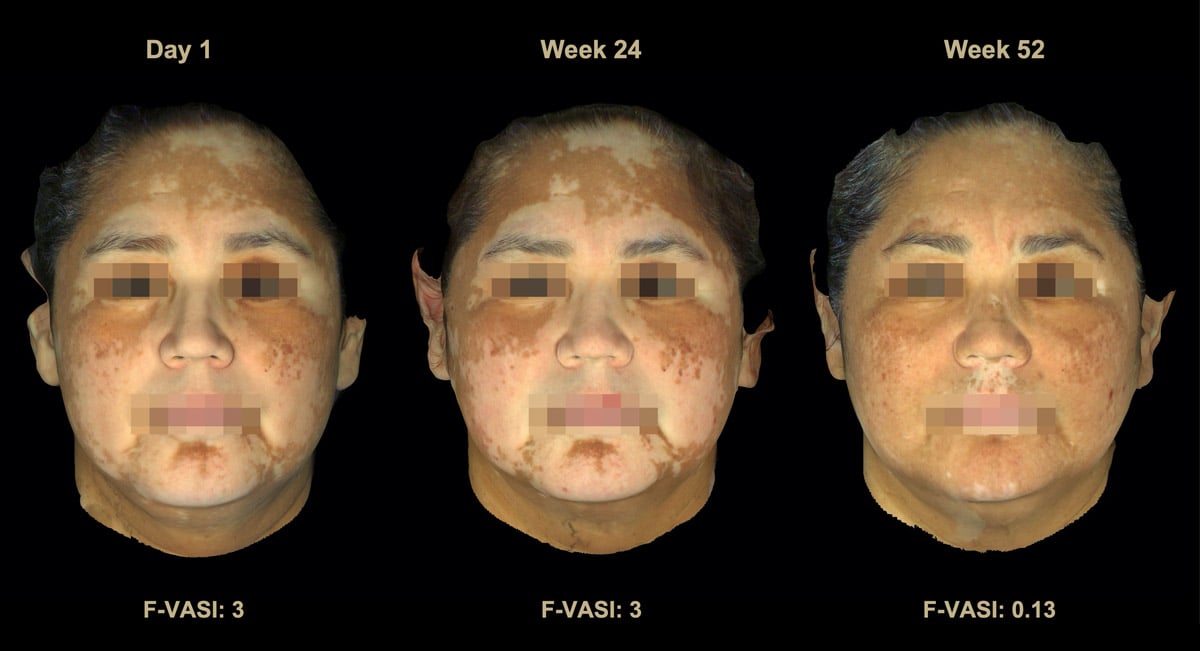
You may ask why we’re talking so much about the results on the face, while the cream was tested on all parts of the body. Well, the face is the easiest place to treat, or “lowest bar” for improvement so-to-speak, and patients care a lot about their face, so it’s a great location to measure early treatment responses. The good news is that patients also saw improvement on non-facial areas as well, up to 45% of patients getting at least 50% better on their body. As with most other treatments, hands and feet were the areas that saw the smallest improvement, although they did get some pigment back as well.
Because of these results, Incyte has decided to seek FDA approval for ruxolitinib cream so that it can be prescribed by doctors and filled at local pharmacies. This will require positive results from a Phase III clinical trial conducted in even more patients. In the Phase II trial, the 157 patients were randomized into 5 groups of about 30 each to test different doses of the drug. Now Phase III will test only the highest dose of the drug (since that appeared to be safe in Phase II) in 2 studies with 300 participants each so that a lot more patients will get the drug to better measure the improvement. This study has already started and will be enrolling patients from all over the world, this time as young as age 12 – so more opportunities for more people! Sign up for our newsletter below to receive updates on clinical trials when they’re available.
Subscribe for Clinical Trials Updates