I am excited to announce outstanding results of the first large, randomized clinical trial to test a treatment for vitiligo! For some background, we and a few others reported that the JAK inhibitors tofacitinib and ruxolitinib were effective treatments in a small number of patients with vitiligo. The rationale for using these treatments was based on the cytokine signaling pathways responsible for driving vitiligo, which you can read more about in detail here: https://www.umassmed.edu/vitiligo/research/adaptive-immunity/
These inhibitors were used both orally and topically in a few patients, and Incyte was the first company to initiate a clinical trial in vitiligo to test these treatments formally. As a quick reminder, here is the blog post I wrote in the summer of 2017 announcing the study:
https://www.umassmed.edu/vitiligo/blog/blog-posts1/2017/06/topical-jak-inhibitor-shown-to-be-effective-for-facial-vitiligo/
The trial was started in the spring of 2017 and recruited 157 participants, completing enrollment for the study in the fall of 2018. During the first 6 months, participants were randomized to placebo or 4 different treatment doses of the drug. For the next 6 months, those who were initially randomized to placebo were re-randomized to one of the higher doses of the drug. For the following year, all participants were given the highest dose of the drug and allowed to use phototherapy if they wanted.
At the meeting in Milan on Saturday morning, we reported the results from the first 6 months of treatment, and they were outstanding! 139 out of 157 participants completed this portion of the study, and these included an equal representation of men and women, with a mean age of 48 years old. Participants were well represented from the medium skin tones, but those with very light skin and very dark skin were under-represented. The company wants to improve the representation in these categories in future studies. Most subjects had been on some prior treatment without satisfactory results.
Within 8 weeks, participants randomized to the treatment were already seeing a significant improvement, particularly on the face (and some just after 4 weeks). There was clear separation from placebo by 12 weeks. By the end of the 6-month period, 50% of subjects on the 2 highest doses of treatment had achieved a F-VASI-50, meaning they had 50% of the pigment back in their spots on their face, and this was expected to continue with further treatment. Just over 30% of participants on the highest dose had even achieved a F-VASI-75, meaning they had at least 75% of their pigment back on their face in 6 months, which is fast. The placebo response was very low, with only 3% achieving an F-VASI-50, and nobody achieving F-VASI-75. While not reported in the short presentation, subjects saw improvement on non-facial areas of their body as well. Side effects of using the medication consisted primarily of increased acne in 10-15% of participants, while other reported side effects were not more common in patients using the drug compared to those using the placebo, and very few discontinued the treatment because of any side effects.
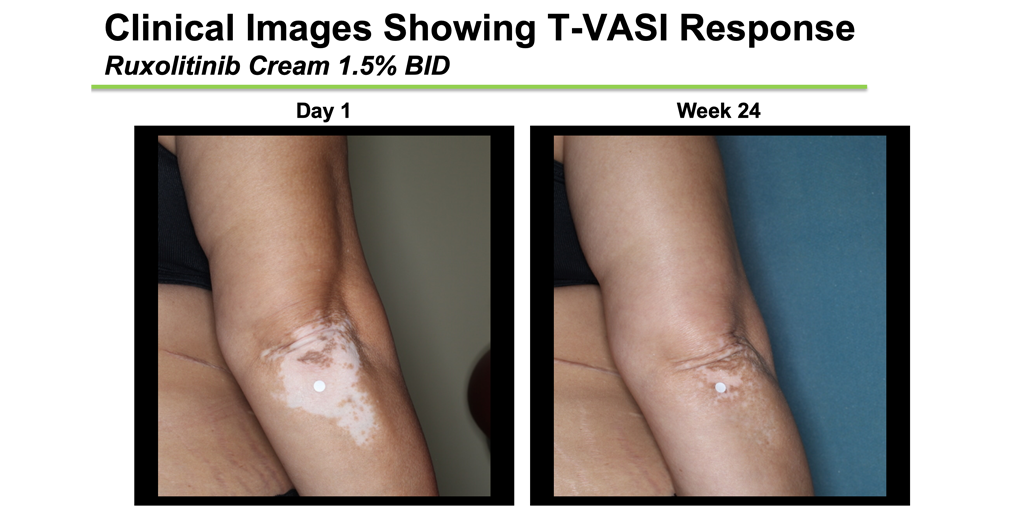
These results are really good, and we fully expect that the 1-year results will be even better since the trajectory of improvement was still increasing at the 6-month time point. Because of these promising results, there is word that Incyte is strongly considering a worldwide Phase III study for this drug, which would allow them to seek FDA-approval to market the drug for vitiligo patients. If this happens, it would be the first FDA-approved medical treatment to reverse vitiligo. Keep your eyes open for more results and announcements, this is only the beginning!
We will soon be announcing a new clinical trial that we will start at UMass and other sites around the world. Expect a newsletter VERY soon with this information.
If you are interested in Incyte Phase II Clinical Trial Results Full Report presented at the World Congress of Dermatology in Milan, Italy, you can download it here.