Our Research
Misregulation of the cell division cycle is one of the hallmarks that defines all cancer cells. The cell cycle is regulated at three levels–transcription, protein degradation, and phosphorylation–all of which are important to control cell-cycle events. Our goal is to understand how these different modes of regulation are integrated to generate a robust control network. A better understanding of this network will enable us to identify regulatory steps that can be targeted to inhibit proliferation and develop more effective cancer therapies.
Phosphoregulation of the cell cycle
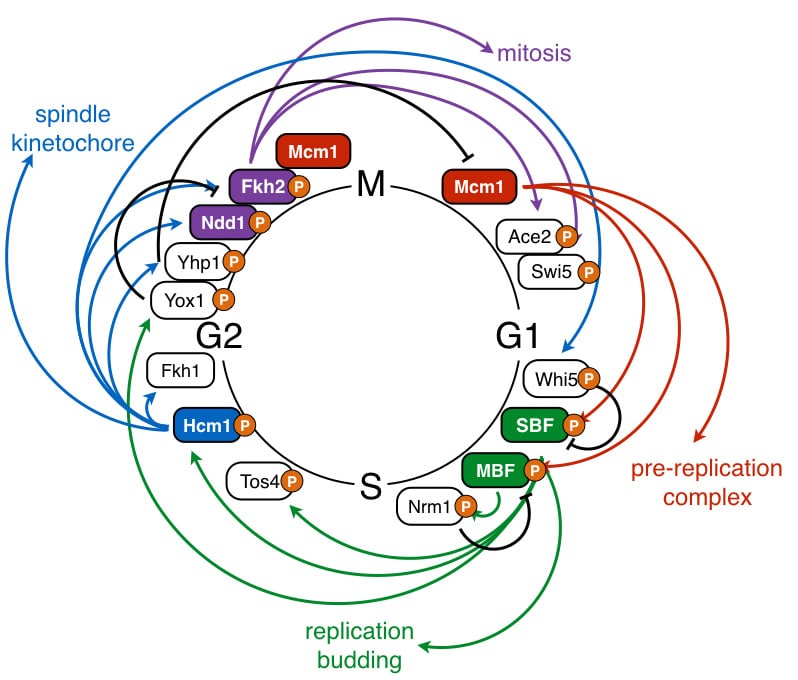 Cyclin-dependent kinases (CDKs) phosphorylate a wide array of proteins to coordinate diverse cellular processes with cell division. Although hundreds of CDK substrates have been identified, for most substrates it is not known how phosphorylation regulates protein function. Our lab studies how CDK phosphorylation controls the activity of cell cycle-regulatory proteins (Landry et al, EMBO J, 2014; Marceau et al, eLife, 2019). We utilize systems-level genetic approaches to determine how CDK phosphorylation of substrates impacts cellular physiology (Conti, Ghizzoni, et al, PLoS Genetics, 2022), and have developed high-throughput methods to decode multisite phosphorylated domains to identify the critical phosphorylation sites that impact protein function (Conti et al, Nat Commun, 2023).
Cyclin-dependent kinases (CDKs) phosphorylate a wide array of proteins to coordinate diverse cellular processes with cell division. Although hundreds of CDK substrates have been identified, for most substrates it is not known how phosphorylation regulates protein function. Our lab studies how CDK phosphorylation controls the activity of cell cycle-regulatory proteins (Landry et al, EMBO J, 2014; Marceau et al, eLife, 2019). We utilize systems-level genetic approaches to determine how CDK phosphorylation of substrates impacts cellular physiology (Conti, Ghizzoni, et al, PLoS Genetics, 2022), and have developed high-throughput methods to decode multisite phosphorylated domains to identify the critical phosphorylation sites that impact protein function (Conti et al, Nat Commun, 2023).
Rewiring of the cell cycle in response to environmental stress
When cells are exposed to an environmental stressor, the cell cycle is delayed until they adapt to their new environment. Our lab is interested in understanding how stress response pathways interface with the cell cycle-regulatory network to pause the cell cycle as cells adapt. We study how conserved stress response pathways inactivate cell-cycle regulatory transcription factors to downregulate gene expression in response to stress (Arsenault, Roy et al, Mol Biol Cell, 2015; Leech, Flynn, et al PLoS Genet, 2020). We are also interested in understanding how stress response pathways cooperate to arrest the cell cycle (Flynn & Benanti, PNAS, 2022), and in the importance of crosstalk between these pathways for survival in stressful environments.
Cell-cycle regulation by the ubiquitin proteasome system

Protein degradation via the ubiquitin-proteasome system (UPS) is essential for cells to grow and divide. Consistent with this role, numerous ubiquitin ligases (E3s) that target proteins to the proteasome for degradation, as well as deubiquitinating enzymes (DUBs) that antagonize E3 function, are mutated in cancer cells. However, the targets of most of these enzymes remain unknown. We use budding yeast as a model system to determine how conserved E3s and DUBs recognize and select their substrates, and to develop proteome-wide approaches to identify targets of these critical enzymes (Benanti et al, Nat Cell Biol, 2007; Mapa et al, Mol Biol Cell, 2018). We are also interested in understanding the cell-cycle regulatory roles of other UPS proteins that are misregulated in cancer cells (Arsenault et al, G3, 2021).