Local Ca2+ signaling
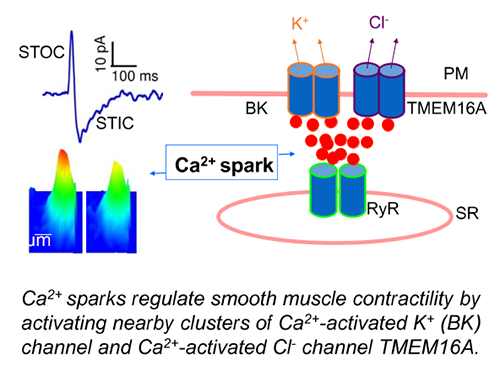
Ca2+ is a universal signaling ion for both life and death. Its concentration within the cell is precisely controlled and regulated in time and space. We have been studying localized, short-lived Ca2+ transients (Ca2+ sparks) that result from the opening of a few clustered ryanodine receptors (RyRs) in the membrane of the sarcoplasmic reticulum (SR). These local Ca2+ signals are the elementary events of precipitating global changes of Ca2+ in striated muscles. In smooth muscle from airways, corpora cavernosa, and some blood vessels, Ca2+ sparks act in their own right to turn on a cluster of big-conductance Ca2+-activated K+ (BK) channels and Ca2+-activated Cl- (Cl(Ca)) channels in the vicinity of release sites (Bao et al. 2008; Lifshitz et al., 2011). Activation of these two types of channels produces spontaneous transient outward currents (STOCs) and spontaneous transient inward currents (STICs), respectively, which regulate the activities of voltage-dependent Ca2+-permeable channels. We discovered that Ca2+ sparks function as stabilizers of membrane potential and control the contractile state of airway smooth muscle (ZhuGe et al. 2010), and Cl(Ca) channel TMEM16A is up-regulated in a mouse model of chronic asthma (Zhang et al. 2013). We aim to understand the mechanisms by which Ca2+ sparks activate BK and TMEM16A Cl(Ca) channels, investigate the structure and function of TMEM16A, and determine the roles of Ca2+ spark signaling in asthma.
Neurotransmitters transmit the signals from a neuron to its target neurons or non-neuronal cells. It is known that neurotransmitter release is triggered by Ca2+ influx through voltage-dependent Ca2+ channels. However, it remains elusive how Ca2+ releases from internal Ca2+ stores affect neurotransmitter release. Using adrenal chromaffin cells, we discovered that these cells predominantly express RyR2, the cardiac isoform of RyR, and their opening generates localized Ca2+ events, designated as Ca2+ syntillas (De Crescenzo et al. 2004). We also found that Ca2+ syntillas do not trigger catecholamine release (ZhuGe et al. 2006) as current neurotransmitter release dogma would predict. We further demonstrated that Ca2+ syntillas suppress spontaneous catecholamine release (Lefkowitz et al. 2009). Also, action potentials at physiological frequency release catecholamine, in large part due to their inhibition of Ca2+ syntillas (Lefkowitz et al., 2014). So our studies have revealed a new mechanism for the regulation of neurotransmitter release. Since catecholamine mediates many fundamental physiologic responses such as the “fight or flight” stress response, studying this mechanism could produce a new understanding of these essential biological functions.