
A new National Institutes of Health (NIH) award will help support the continued study of vesicle tethering and membrane fusion in exocytosis for five more years at UMass Chan Medical School.
Mary Munson, PhD, professor and vice chair for diversity in the department of biochemistry & molecular biotechnology, and assistant vice provost for health equity, received the NIH award.
“We are really interested in the deep fundamental mechanistic details of how protein cargos get from the inside of the cell to the outside of the cell and how others are inserted into the cell membrane,” Dr. Munson said. “The grant supports our projects that are really digging deep in the machinery of exocytosis.”
The Munson lab is focused on illustrating the molecular mechanisms of membrane fusion regulation. The NIH grant is for $3.3 million over five years.
In eukaryotic cells, intracellular cargo, like proteins, are packaged into vesicles which get transported to the plasma membrane. The vesicle membrane is then fused to the cell’s membrane to deliver the cargo. A large protein complex called exocyst fuses the vesicles in the right places on the plasma membrane.
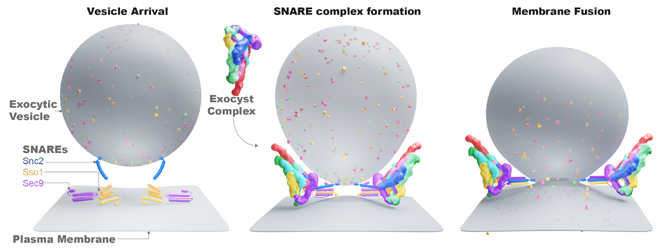
The processes of vesicular transport and membrane fusion are crucial for cellular morphology, growth, movement and secretion, including hormone release and neurotransmission. But, according to Munson, the processes aren’t yet fully understood. With the grant, the Munson lab aims to understand what happens in the specific point in time before fusion takes place and discover how vesicle targeting and membrane fusion is achieved.
“We’re working on how the exocyst protein complex waits for a signal to fuse the vesicle to the membrane. And that’s when the magic happens. We have an idea of what the mechanism might look like, but we don’t know how it works yet,” Munson said. “Exocyst is absolutely required for the vesicle to fuse and deliver its cargo. The cell won’t grow, won’t divide and won’t talk to other cells without this mechanism.”
Munson hopes her research will also lead to the development of tools to study how the molecular mechanism regulates secretion in all eukaryotic cells. Future research areas will focus on developmental and neurological disorders that may arise when exocyst is disrupted.
This spring, Munson was elected to serve as president of the American Society for Cell Biology (ASCB). She will serve as president-elect on the ASCB executive committee starting next year and will assume the role of the society’s president in 2025.

