DNA Replication
How do DNA polymerase clamps and their binding partners drive DNA replication?
Chromosomal DNA replication requires that DNA polymerases be tethered to ring-shaped sliding clamps that encircle the DNA and allow for high-speed, processive replication. These clamps create a sliding platform for the action of scores of enzymes and other proteins to scan and act on DNA. The clamp acts as a central hub to organize the activities of enzymes in the various cellular pathways of DNA replication, DNA repair, cell cycle control, chromatin structure, and apoptosis.
However, how these various activities are coordinated spatially and temporally is unknown. We want to know the cellular, molecular, and structural determinants for this coordination.
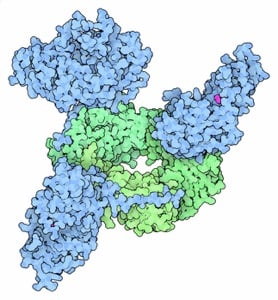
The Kelch Lab discovered how a hypomorphic disease-causing mutation in the human sliding clamp causes specific perturbations to a subset of clamp-binding partners (see Duffy et al JMB 2016). More recently, we reported the discovery of another mutation that results in PCNA-Associated DNA Repair Disorder (Magrino et al JBC 2022; see story in ASBMB Today here). Our multi-disciplinary studies have revealed that both mutations cause a stability defect in PCNA, and that this stability defect may be the molecular basis for disease.
How are clamps loaded onto DNA?
Sliding clamps are loaded onto DNA by the clamp loader complex, a pentameric assembly of proteins of the AAA+ family of ATPases.
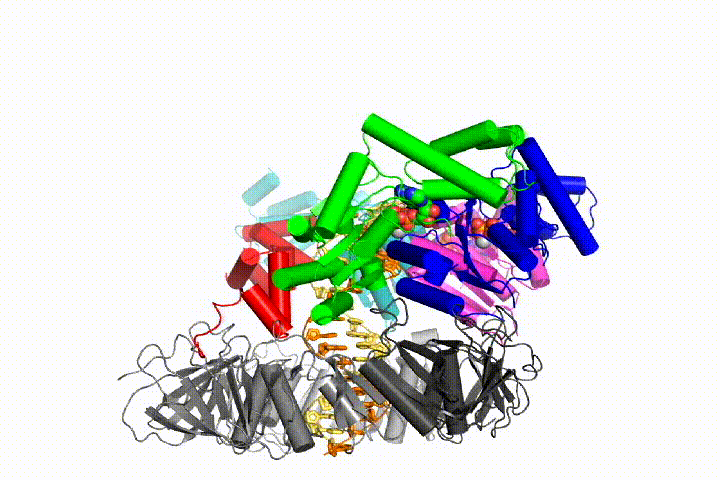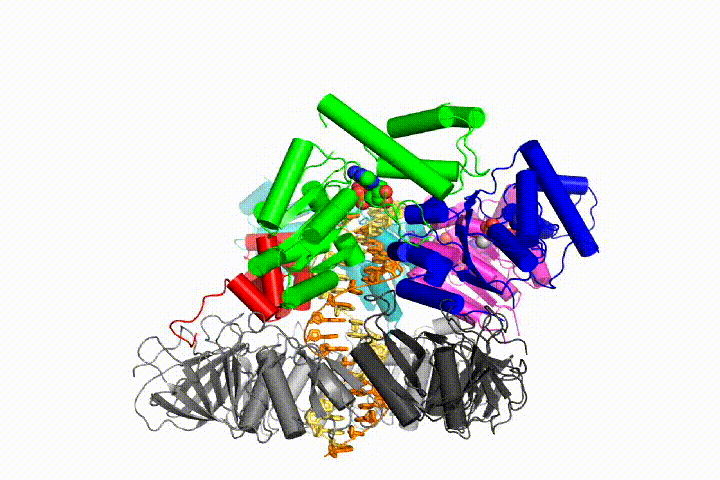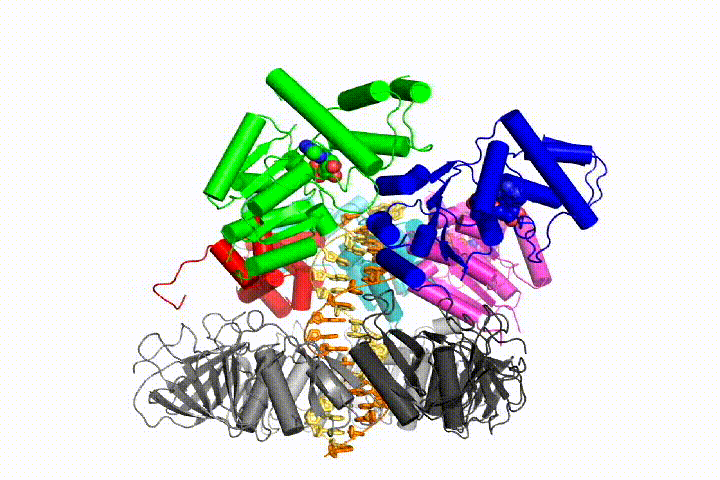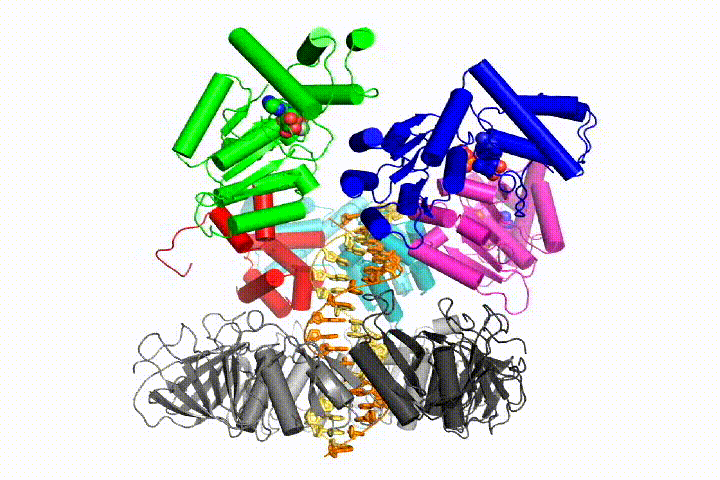
We want to understand the function and mechanism of clamp loaders in atomic detail. Our structures of the T4 bacteriophage clamp loader in complex with primer-template DNA and the sliding clamp have suggested a working model for the clamp loader mechanism as shown below (see Kelch et al Science 2011):
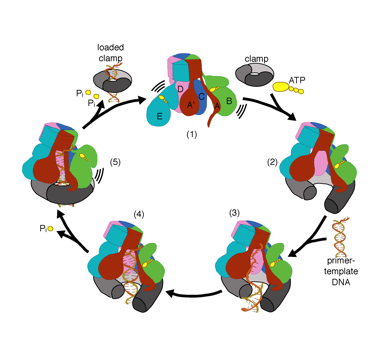
Our model predicts that ATP hydrolysis begins at the end of the AAA+ spiral and proceeds in a sequential fashion up the spiral, resulting in the ejection of the clamp loader from the DNA and the closed clamp. Our hypothesis is illustrated in this animation of three consecutive ATP hydrolysis events:
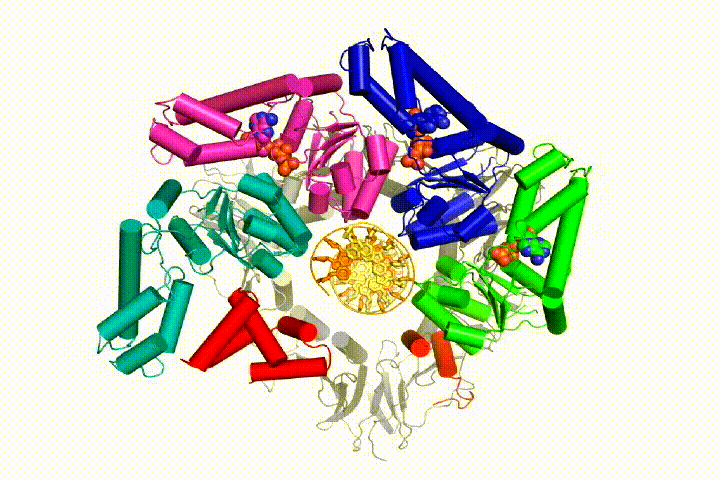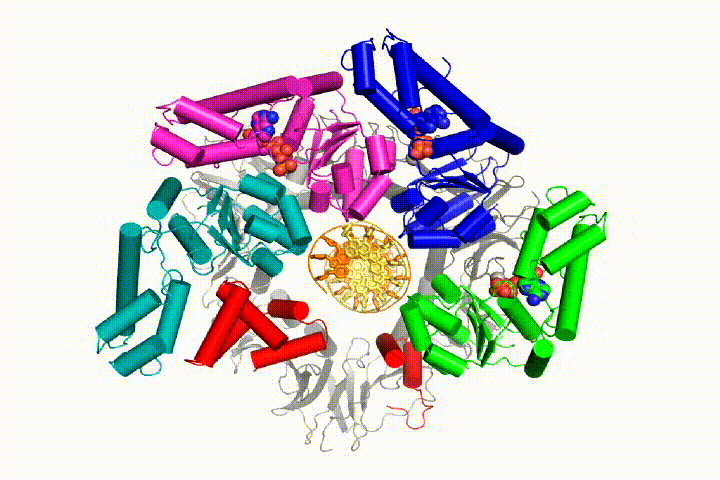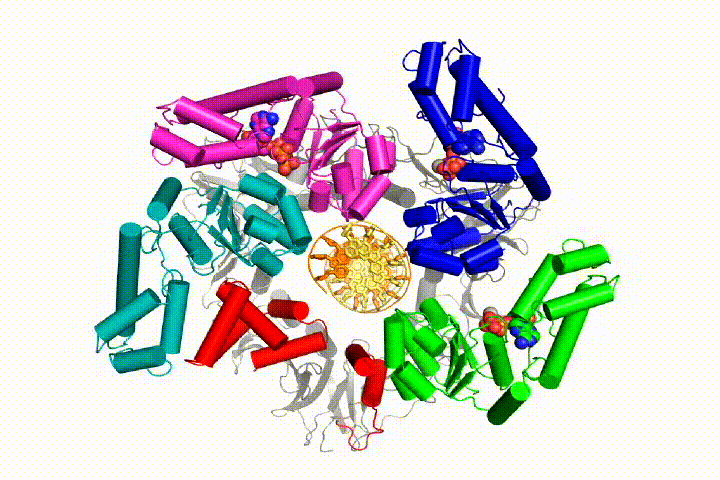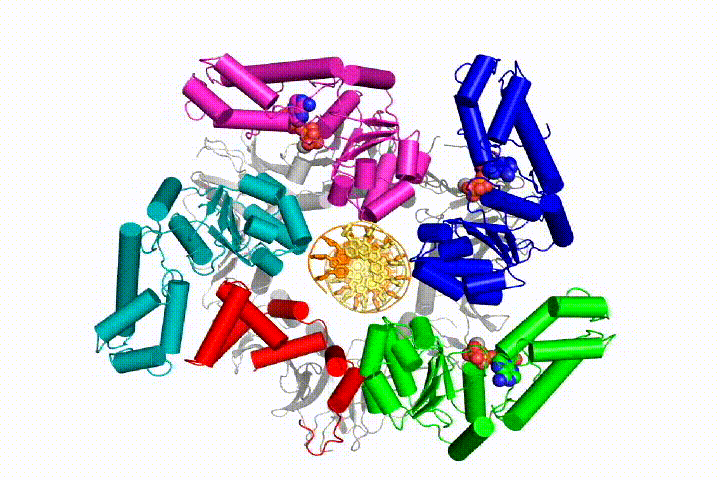
More recently, we revealed the structural mechanism of clamp loading in unprecedented detail. Our cryo-EM structure of the human clamp loader RFC bound to PCNA revealed an early intermediate where the PCNA is closed and RFC is in an auto-inhibited conformation (Gaubitz & Liu et al PNAS 2020). This conformation is similar to a crystal structure of the yeast clamp loader, suggesting that this auto-inhibited state is conserved across eukaryotes.
We reported a detailed mechanism for the yeast clamp loader, with seven structures of clamp loading intermediates (Gaubitz et al, eLife 2022). We find a crab-claw mechanism for opening the clamp, which explains how the clamp loader achieves preference for its substrates. This allows DNA to directly bind into the large cleft, which induces clamp closure. We observe tight interactions at the ATPase active sites upon clamp closure, explaining how the clamp and DNA synergistically activate ATP hydrolysis and clamp release. We also reported the discovery of a second DNA binding site on the exterior surface of the eukaryotic clamp loader (Liu & Gaubtiz et al eLife 2022). We reveal that both the external and internal DNA binding sites have the ability to partially unwind DNA without hydrolyzing ATP. Furthermore, we find that the two DNA binding sites cooperate to enable the clamp loader to bind to DNA at a nick or a small single-stranded gap, which are common intermediates during DNA repair. Finally, we show that yeast with a disrupted external DNA binding site have a defective DNA damage response, illustrating that this site is important for physiological function.
What about alternative clamp loaders?
Beyond the clamp loaders described above, Eukaryotic organisms have an array of alternative clamp loaders that are used in distinct pathways such as DNA damage and checkpoint control, sister chromatid cohesion, and recombination (see Kelch Biopolymers 2016). These clamp loaders have been suggested to be important targets for novel cancer therapeutics. Despite differing from the replicative clamp loader by just one subunit in the pentameric assembly, the alternative clamp loaders exhibit significant functional and mechanistic differences. For example, the Rad24 clamp loader binds a different sliding clamp, the ’9-1-1 complex’ (Majka & Burgers, PNAS 2003), and functions at the 5’ DNA junction, the opposite polarity to that of the replicative clamp loader (Ellison & Stillman, PLOS Bio 2003).
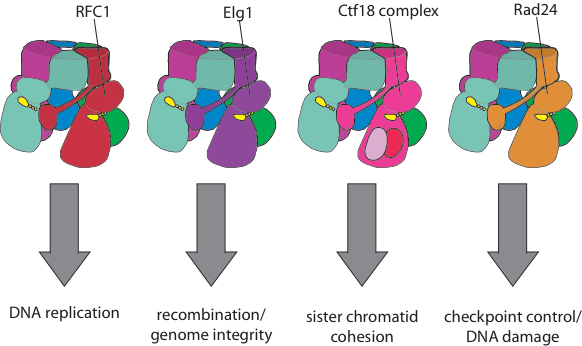
Our goal is to determine the structural and biochemical basis for the mechanistic and functional differences of the alternative clamp loaders from their replicative clamp loader cousins. We will also understand how alternative clamp loaders are regulated and integrated into the pathways of recombination, sister chromatid cohesion, and DNA repair.
Overall, we hope our studies of clamp loaders in atomic detail will have important implications for understanding DNA replication and other cellular processes. Ultimately, we anticipate using our mechanistic understanding to develop novel nanodevices and small molecule effectors of AAA+ function. We have summarized our understanding of clamp loaders in reviews here, here, and here.
