Type 2 Diabetes Projects with Dr. Jason Kim at UMass Chan Medical School
Role of GRP78 and Unfolded Protein Response in Macrophage Function and Insulin Resistance in Diet-Induced Obesity
We have recently found that obesity-mediated inflammation is associated with M1 polarization of macrophages and increased secretion of inflammatory cytokines and their deleterious effects on skeletal muscle insulin signaling and glucose metabolism (Han, M.S. et al., Science 2013;339:218-222). The 78-kDa glucose-regulated protein (GRP78) is a major endoplasmic reticulum (ER) chaperone, and our previously published work showed that GRP78 modulates unfolded protein response (UPR) and ER homeostasis (Ye, R. et al., Diabetes 2010;59:6-16 and Zhu, G. et al., FASEB J. 2013;27:955-964). Based on these findings, we have recently generated mice with conditional deletion of Grp78 in myeloid cells (Lyz-Grp78-/-), and Lyz-Grp78-/- mice are shown to be protected from obesity-mediated insulin resistance, and the underlying mechanism involves upregulation of ATF-4 and other UPR elements with increased alternatively-activated (M2) macrophages, resulting in increased insulin signaling and glucose metabolism in skeletal muscle (Kim, J.H. et al., FASEB J. 2018;32:2292-2304). Our findings implicate a potential role of ER stress and UPR in macrophage function, and we are continuing to investigate the effects of obesity in macrophages and other immune cells.
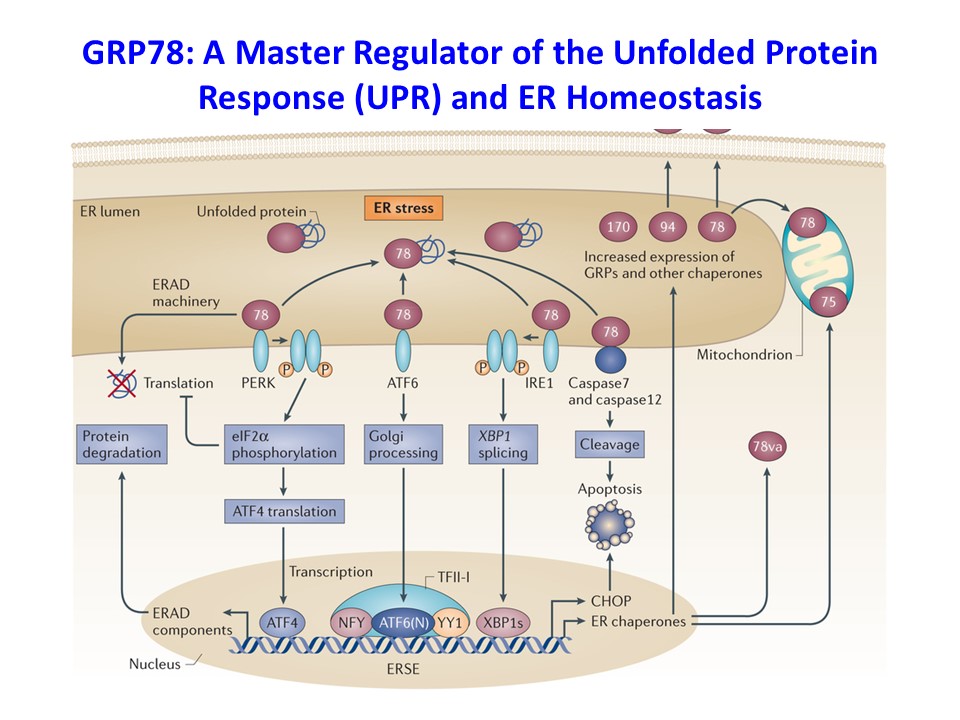
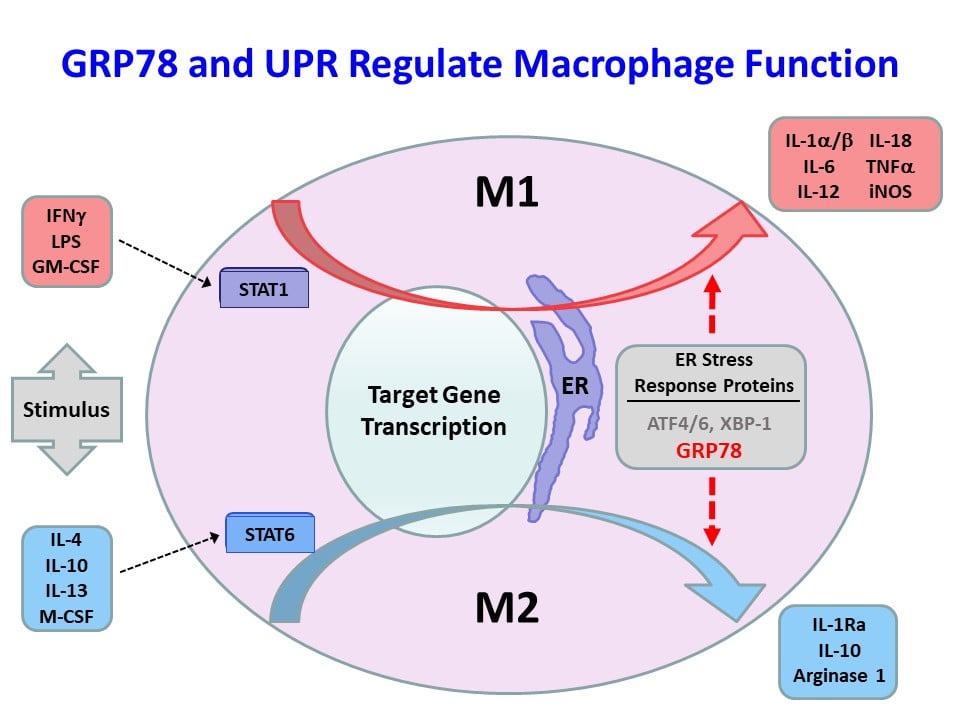
Role of IFNγ and IL-1a in Obesity-Mediated Inflammation and Insulin Resistance
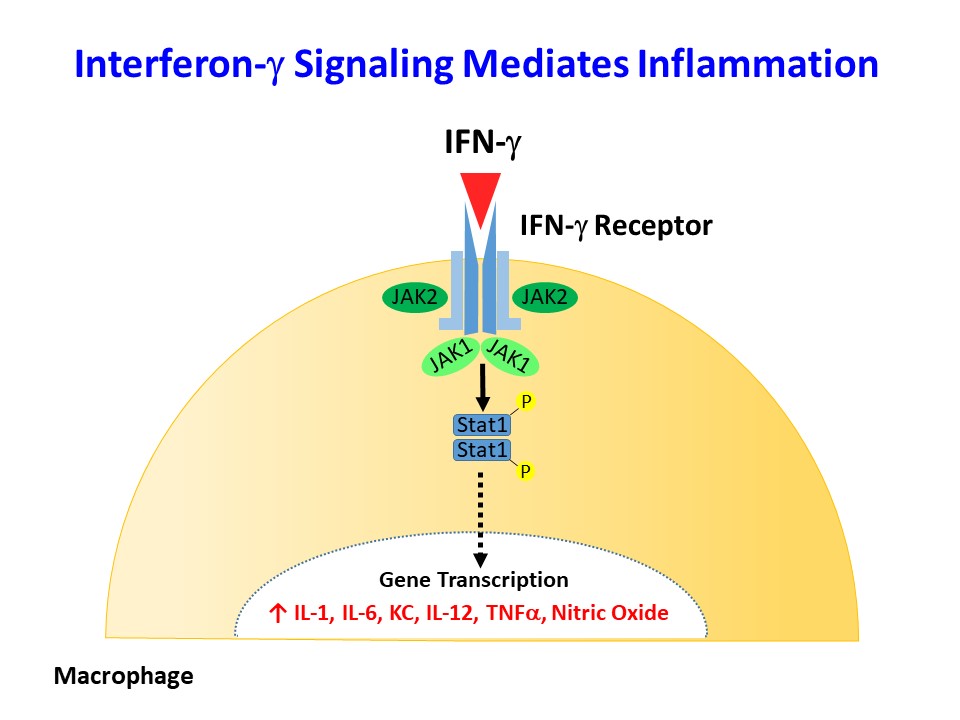
Interferon-γ (IFNγ) and IL-1a are major inflammatory cytokines elevated in obesity, but their role in obesity-mediated insulin resistance and type 2 diabetes is unknown. As a primary modulator of the macrophage phenotype, IFNγ promotes a strong pro-inflammatory M1 macrophage response that correlates with macrophage recruitment to metabolic organs in obesity. We have recently generated mice with conditional deletion of IFNγ receptor (Lyz-IFNγR2 KO) to disrupt the IFNγ signaling in myeloid cells, and we are currently investigating the effects of diet-induced obesity in Lyz-IFNγR2 KO mice. We have also generated mice with conditional deletion of IL-1a in myeloid cells (Lyz-IL1a KO) to determine the effects of myeloid cells lacking IL-1a in obesity-mediated inflammation and insulin resistance.
Role of Anti-Inflammatory Cytokine, IL-10 in Regulation of Glucose Metabolism, Insulin Signaling, and Skeletal Muscle Myogenesis in Obesity and Aging
We have initially made two important discoveries: 1) In diet-induced obesity, inflammation develops in skeletal muscle with macrophage infiltration and locally elevated inflammatory cytokines (Hong, E.-G. et al., Diabetes 2009;58:2525-2535), and 2) cytokines regulate glucose metabolism and insulin signaling in skeletal muscle (Kim, H.-J. et al., Diabetes 2004;53:1060-1067). Based on these findings, we have generated transgenic mice with muscle-selective overexpression of IL-10 (MIL-10), and MIL-10 mice are protected from obesity-mediated inflammation and insulin resistance in skeletal muscle (Dagdeviren, S. et al., Mol. Cell. Biol. 2016;36:2956-2966).
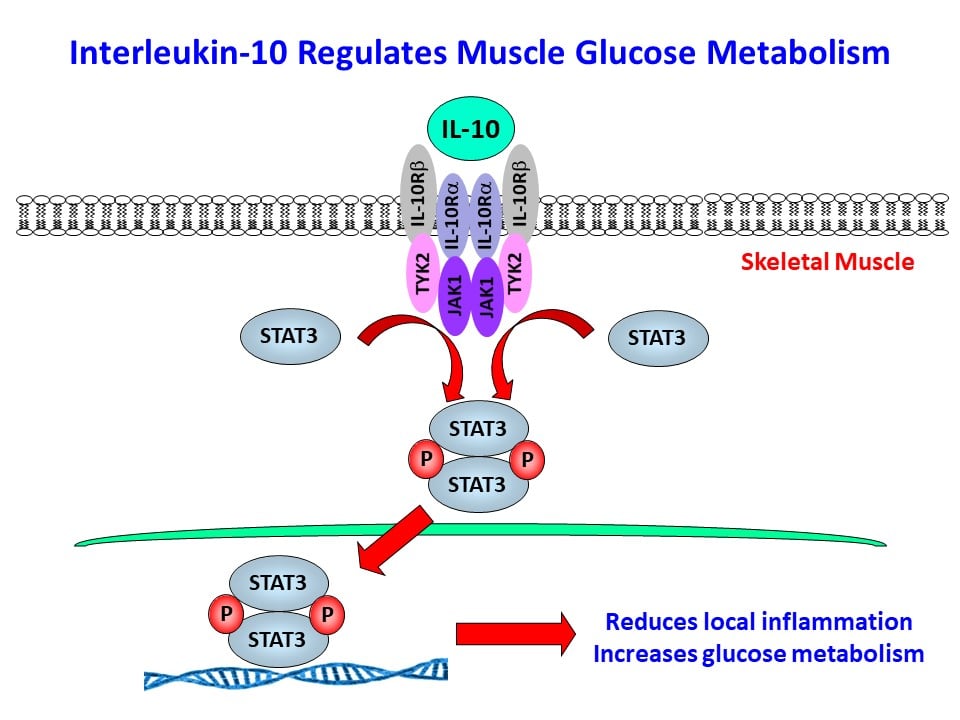
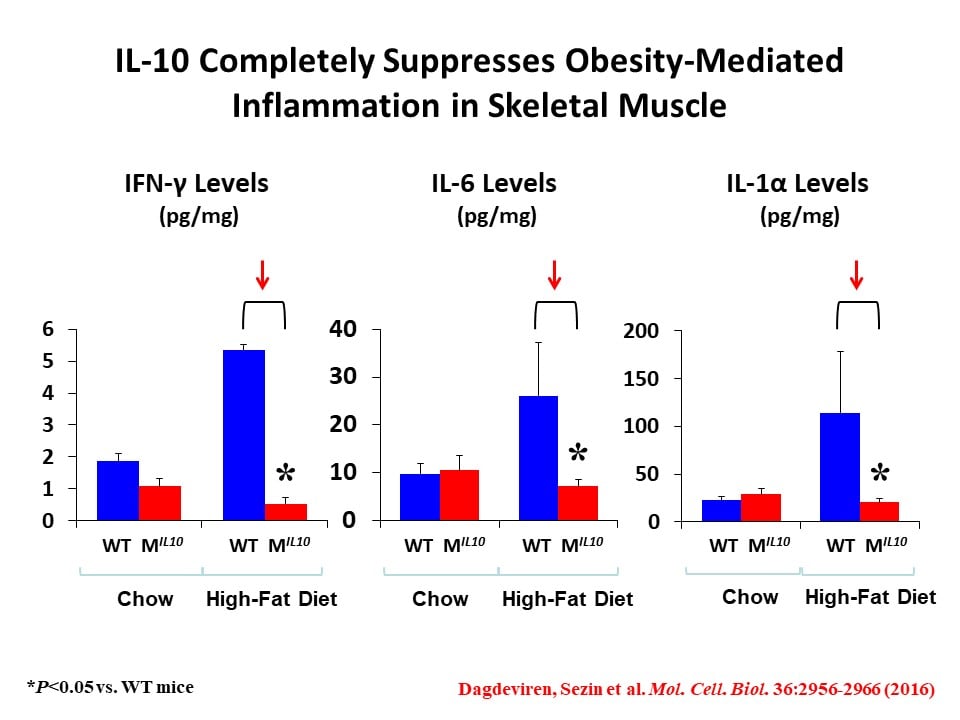
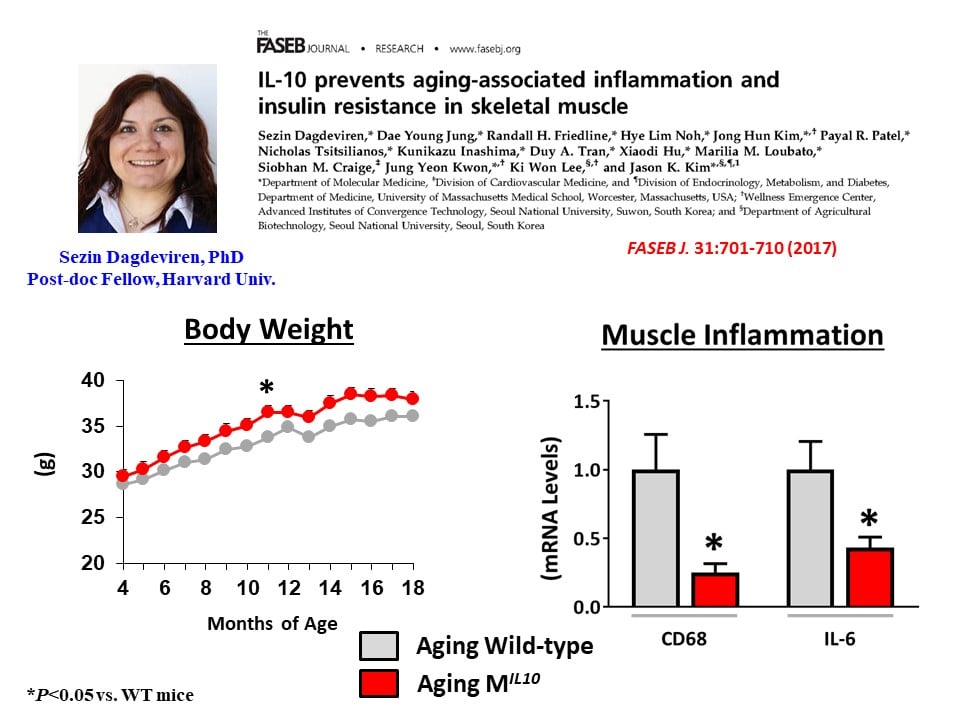
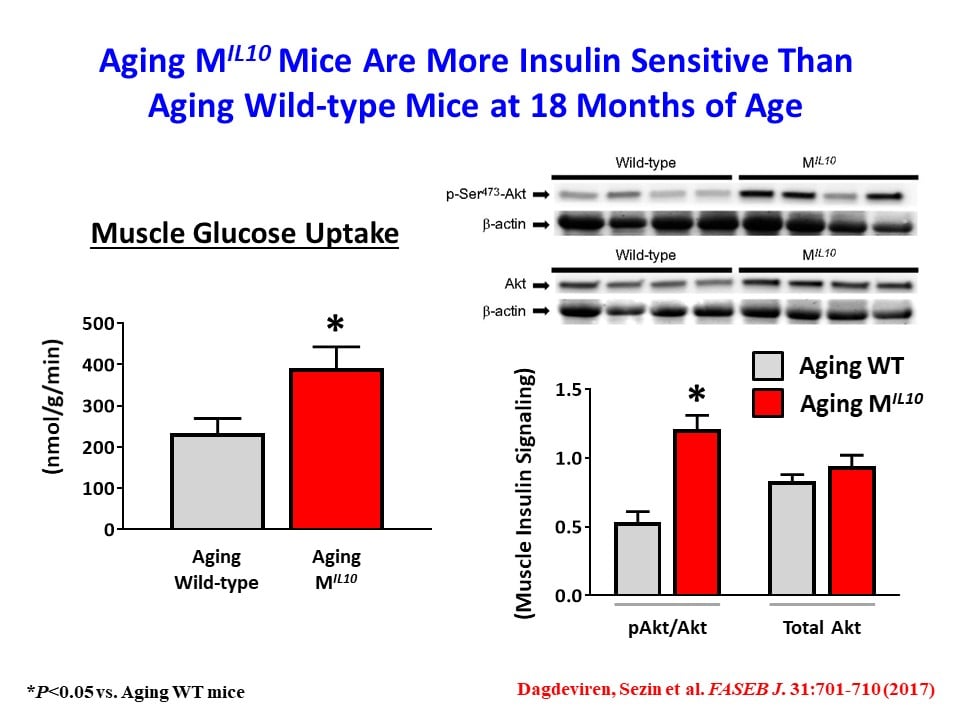
We have also found that aging MIL-10 mice at 18 months of age are more insulin sensitive with increased glucose metabolism than aging wild-type mice, suggesting a potential role of muscle inflammation in aging-associated insulin resistance (Dagdeviren, S. et al., FASEB J. 2017:31:701-710). Interestingly, we have found increased muscle mass in MIL-10 mice, and we are continuing to investigate the molecular mechanisms by which IL-10 regulates muscle inflammation, insulin signaling, and myogenesis and identify potential therapeutic targets for the treatment of insulin resistance (pre-diabetes) in obesity and aging.
Dissecting the Beneficial Effects of Weight Loss on Insulin Sensitivity and Type 2 Diabetes
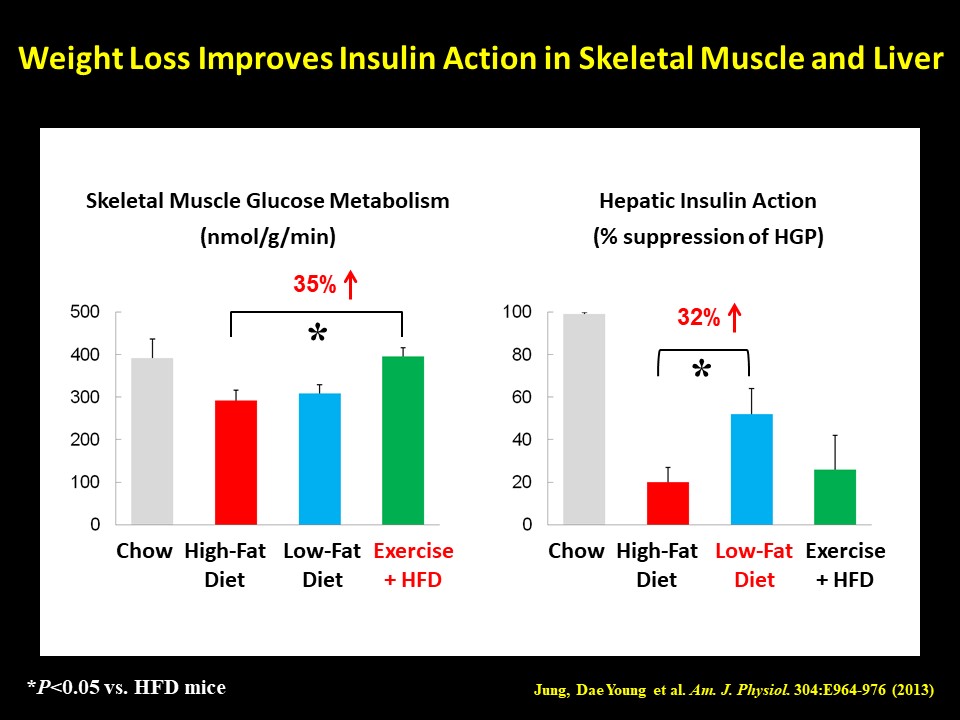
Diabetes Prevention Program studies have demonstrated that a modest weight loss can profoundly improve insulin sensitivity in diabetic subjects. As environmental factors, such as high-fat, high-carbohydrate, and high-caloric diets coupled with reduced physical activity, play a major role in the current obesity pandemic, weight loss by changing various lifestyle factors is a standard nonpharmacological regimen to treat type 2 diabetes. Although the beneficial effects of weight loss on metabolic homeostasis are well established, the molecular mechanism by which weight loss improves insulin sensitivity remains unclear. Our recent study found that a short-term weight loss, mediated by either switching from a high-fat diet to a low-fat diet or engaging in exercise (via cage wheel), caused a dramatic increase in insulin sensitivity in skeletal muscle and liver even though the mice remained relatively more obese than control animals (Jung, D.Y. et al., Am. J. Physiol. 2013;304:E964-E976). Interestingly, there was a weight loss modality-dependent effects on organ-specific insulin action, and this beneficial effect of modest weight loss did not involve attenuation of adipose tissue inflammation. We are currently investigating how weight loss impacts local inflammation and glucose metabolism in skeletal muscle and liver.

Affiliated Centers
Diabetes Center of Excellence at UMass Chan Medical School
Metabolic Disease Research Center