
Finding the best therapy to treat cancer is as important as making an accurate diagnosis, yet when it comes to hereditary BRCA-deficient breast and ovarian cancers, oncologists’ selections sometimes fall short. Their decisions spring from conventional theories suggesting that chemotherapy kills cancer cells by creating double-stranded DNA breaks. The way in which BRCA-deficient cancers typically respond to chemotherapy further feeds this conviction. These cancer cells lack the crucial proteins necessary for DNA repair, therefore they wither and die.
Except of course when they don’t.
Realizing that any patients who don’t respond to the treatment could lose their lives and driven to understand why patients with this BRCA-deficient cancer do not always respond to chemotherapy, Sharon Cantor, PhD, began her investigation.
Rewriting the narrative
Dr. Cantor’s commitment to understanding these cancers stretches back to her postdoctoral days when she discovered that the gene FANCJ behaved similarly to the two BRCA genes that drive hereditary breast and ovarian cancer. Her tenacious study of FANCJ—and its similarities to and differences from the BRCA genes—ultimately revealed that our current conception of how chemotherapy inflicts damage was fraught with gaps.
Leading up to what she described as her “eureka” moment, Dr. Cantor and her team coated BRCA1, BRCA2, and FANCJ-deficient cells with genotoxic drugs, unveiling an eyepopping finding: The FANCJ-deficient cells survived chemotherapy while the BRCA-deficient cancer cells did not.
“When we analyzed the cells looking for an explanation, we found just one difference—the BRCA-deficient cells were riddled with tiny gaps while the FANCJ cells were not,” says Dr. Cantor.
These findings contradict the comparison of chemotherapy to a chainsaw that cuts DNA in half.
“It seems chemotherapy acts more like a woodpecker, poking holes in DNA,” says Dr. Cantor.
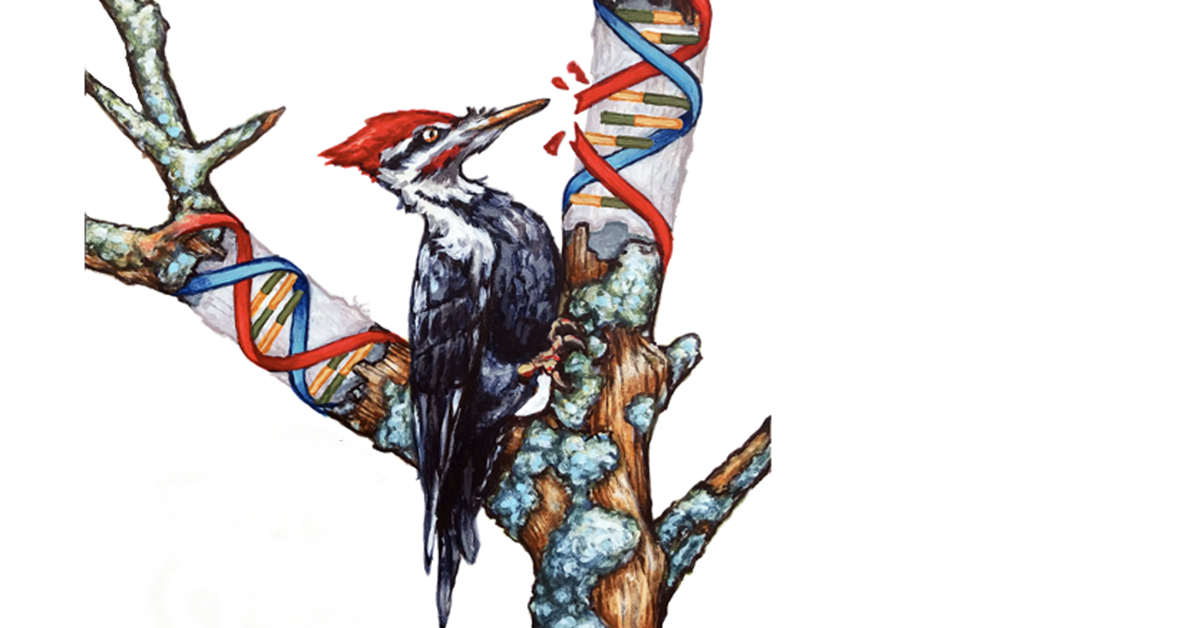
As Dr. Cantor’s team continued to comb through the data, the woodpecker analogy gained traction and revealed that:
1) chemotherapy creates single-stranded DNA gaps;
2) these gaps play a role in initiating cell death; and
3) several days after cell death, double-stranded breaks appear.
Therefore, the gaps, and not the double-stranded breaks, directly result from chemotherapy.
The gap theory gains momentum
Dr. Cantor and her team built a strong case for correlating gaps with chemotherapeutic success, but what about the flipside? As cancer cells develop resistance and chemotherapy begins to fail, are there still gaps?
The answer is a resounding “No.”
“Resistant cells have distinct protein changes indicating they have ‘figured out’ how to suppress the gaps,” explains Dr. Cantor. Luckily, scientists have an antidote. Scientists are already aware of some gap-suppression pathways, and research on one pathway—translesion synthesis—is fairly far along.
“We already know of a translesion synthesis inhibitor that can block this gap-suppressing pathway in cancer cells, while ignoring normal ones. So, it could be a nice selective drug for cancer,” explains Dr. Cantor.
The road ahead
If the gap theory holds up, clinicians will need to change how they make treatment decisions. Currently, clinicians base their recommendations on how likely it is that the cancer will be able to repair double-stranded breaks. As Dr. Cantor and postdoctoral fellow Ke Cong, PhD, explain in their recent Molecular Cell paper, evaluating the cancer cells for gaps—which are present, even prior to treatment, in cancer cells like those with BRCA mutations—may be the best predictor of therapeutic response. Their article also emphasizes that understanding the way single-stranded gaps are both created and suppressed can pave the way to better cancer treatments.
"Considered against the double-stranded break theory, the ‘gap model’ makes the most sense. You don’t have to wiggle the findings to make them fit [the theory],” Dr. Cantor says. “They just do.”