Jul 31, 2024
Celebrating RNA Day with Dr. Thoru Pederson
Read more
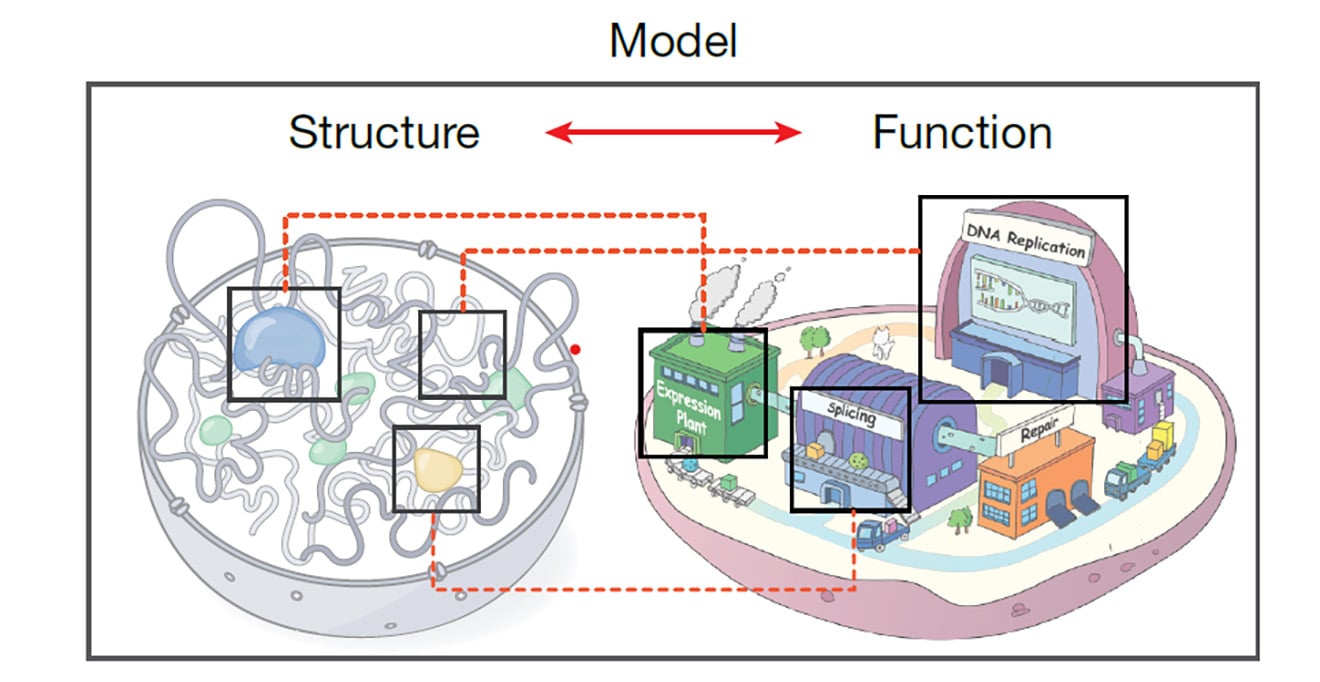
Our mission is to illuminate the molecular mechanisms that govern basic biological processes, with an emphasis on integrative strategies to reveal fundamental mechanistic insights and develop new therapeutic paradigms empowered by a collaborative, diverse & inclusive community.