Clinical Translation
Using our research to help patients
 The testing of gene therapies in large animals is a necessity in the clinical translation process. Our lab focuses on the treatment of large animal models in order to determine how therapeutics behave both in terms of clinical manifestations as well as distribution in the brain and spinal cord. We have a strong focus on optimizing delivery modalities in order to find methods that are both safe and effective, for study animals as well as future human patients.
The testing of gene therapies in large animals is a necessity in the clinical translation process. Our lab focuses on the treatment of large animal models in order to determine how therapeutics behave both in terms of clinical manifestations as well as distribution in the brain and spinal cord. We have a strong focus on optimizing delivery modalities in order to find methods that are both safe and effective, for study animals as well as future human patients.
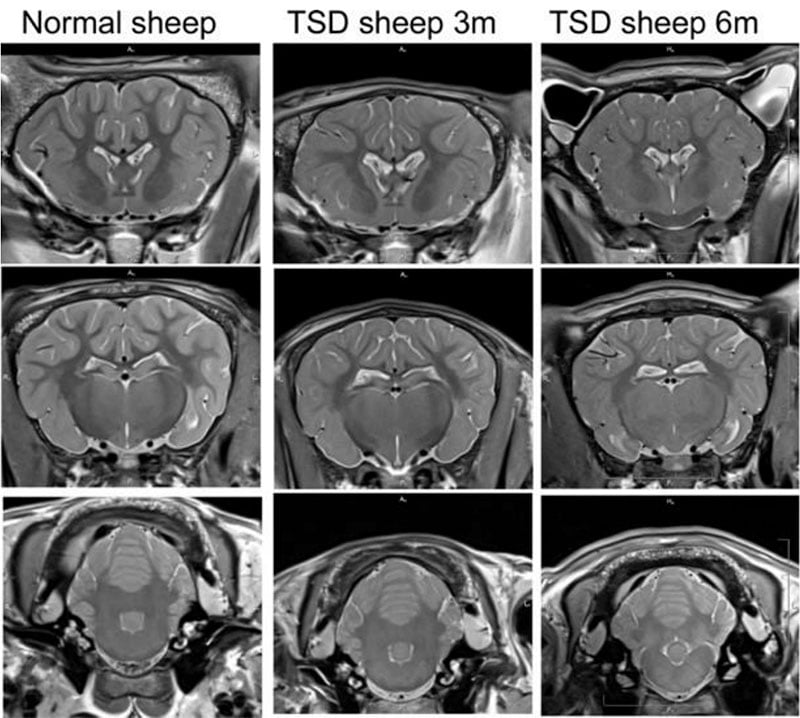
Our lab has studied numerous ways to deliver gene therapies for Tay-Sachs disease to the Jacob sheep. While all delivery methods showed improvements in lifespan and clinical manifestations, we saw that delivery directly into the cerebrospinal fluid (CSF) of the animal resulted in the most dramatic improvements. Our lab went on to optimize the technique by which delivery to the CSF would take place. We found that some methods were less than optimal, due to factors such as tissue damage and loss of therapeutic. In collaboration with researchers and surgeons at UMass Chan, our lab developed two separate techniques that safely deliver the requisite payload of therapeutic. The first technique targets the cisterna magna, while the second method combines intrathecal and intrathalamic delivery.
As a result of these studies, UMass Chan (specifically in conjunction with the Sena-Esteves lab) launched a first in human, compassionate use trial in a 30-month-old child with TSD (cisterna magna delivery). A second, 7-month-old child was subsequently treated (intrathecal/intrathalamic delivery). Both procedures were well tolerated and the patients continue to be monitored for both adverse and therapeutic effects. This is especially significant in the case of injection into the thalamus, which had never been tried in humans. The gene therapy program for Tay-Sachs has since been licensed by Axovant Gene Therapies, with Phase I/II clinical trials upcoming.