Senescence in Stem Cell Self-renewal and Aging
Aging is a complex process in which gradual deterioration of physiological function involves virtually all cells and tissues in an organism. This functional decline is associated with a diminished capacity to maintain normal tissue homeostasis and a reduced regenerative capacity of tissues in response to injury in old organisms. Tissue-specific or adult stem cells are capable of self-renewal to preserve stem cell pools and differentiation into a variety of effector cells. These defining properties of stem cells are essential for the normal homeostatic maintenance and regenerative repair of tissues throughout the lifetime of an organism. With advancing age, the self-renewal capacity of stem cells invariably declines, eventually leading to the accumulation of unrepaired, damaged tissues in old organisms. The precise mechanism underlying age-dependent decline of stem cell self-renewal is largely unclear, but is fundamentally important to our understanding of aging.
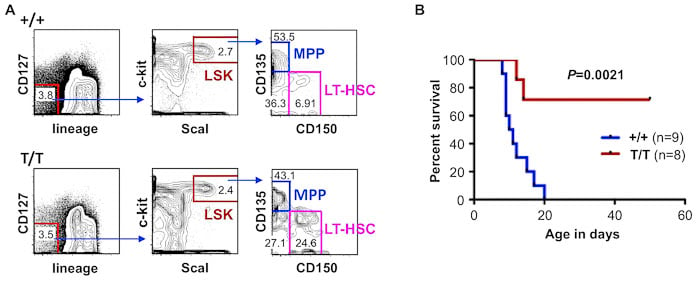
We are interested in the role of senescence in the age-dependent decline of the renewal capacity of stem cells and aging in general. For example, our ongoing studies have found that long-term hematopoietic stem cells (LT-HSC), which give rise to all lineages of blood cells, increase in Smurf2-deficient mice. Furthermore, we have found that Smurf2-deficient HSCs outperform wild-type HSCs in rescuing lethally-irradiated recipient mice in bone marrow serial transplantation, suggesting that Smurf2-deficient HSCs have enhanced renewal capacity. We are investigating the underlying mechanism and asking whether senescence plays a role in HSC self-renewal during aging.
As aging is accompanied by increased susceptibility to all major chronic diseases, including cardiovascular disease, cancer, Alzheimer’s disease, diabetes, arthritis and osteoporosis, we are taking a systematic approach to analyze the impact of senescence on stem cell self-renewal in various tissues/organs, including bone, gut, skin, pancreas, brain and kidney. Understanding the underlying mechanisms of age-associated decline in the renewal capacity of stem cells will help us gain better understanding of aging.
 |
|
Increased long-term hematopoietic stem cell (LT-HSC) population and enhanced self-renewal of HSC in Smurf2-deficient (T/T) mice. (A) Flow cytometry analysis of different stem and progenitor populations. LSK: progenitor population that is negative for lineage makers and high with ScaI and c-Kit; MPP: multipotent progenitors. (B) Increased survival of lethally-irradiated recipient mice after receiving donor bone marrow from Smurf2-deficient mice in serial transplantation. |
