Research
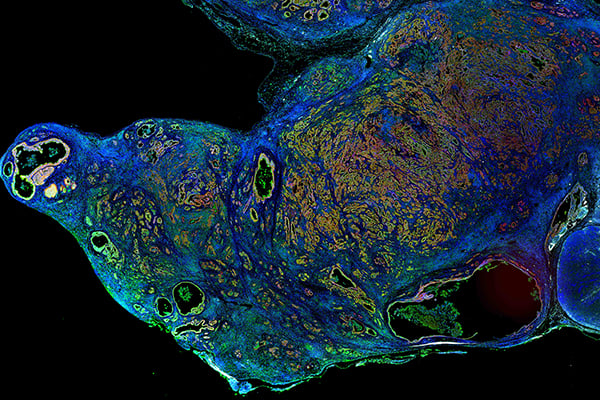
Calcium pathway effectors drive Pancreatic Cancer disease progression
Our recent work established that PTHrP is an important mediator of pancreatic cancer tumor growth and metastasis. In subsequent studies, we found that PTHrP activates intracellular Calcium signaling, which is important for regulating hybrid or partial epithelial-to-mesenchymal (EMT) phenotypes. We have since identified downstream effectors of calcium-driven disease progression and are building new mouse model to test their roles in tumorigenesis in vivo.
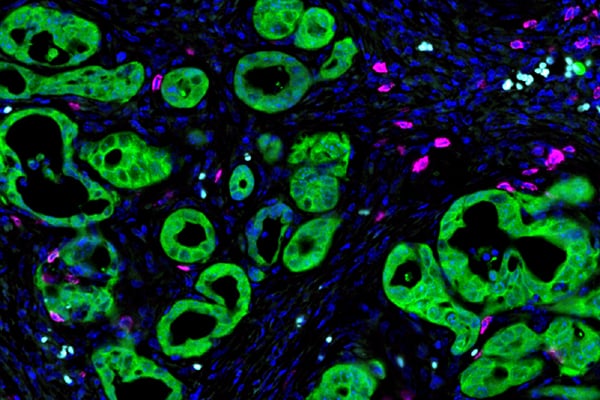
PTHrP as an organizer of an immunosuppressive tumor microenvironment
The vast majority of cells within a pancreatic cancer tumor are of stromal origin and are comprised of fibroblasts, endothelial cells, and various immune cell compartments. A portion of these cells are immunosuppressive and aid the tumor cells in evading the immune system, one of the major reasons that immunotherapy has performed so poorly in pancreatic cancer patients. We have found that the deletion of PTHrP in pancreatic cancer mouse models results in a less immunosuppressive local immune microenvironment by reducing the recruitment of Myeloid-Derived Suppressor Cells (MDSCs) into tumors. Thus, anti-PTHrP therapies being developed by the lab may synergize with current immunotherapy regimens and allow the immune system to better target tumor cells.
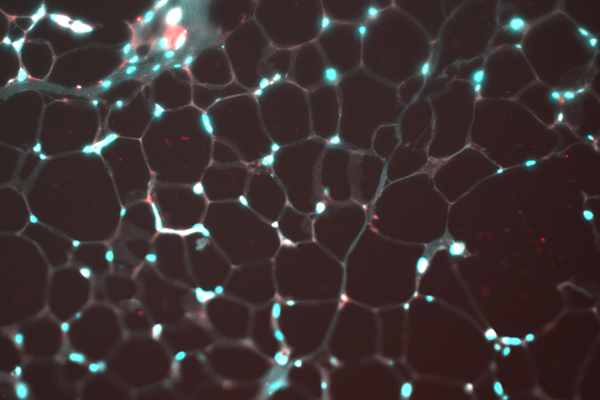
Identifying novel tumor-derived factors that drive cancer cachexia
One of the hallmarks of pancreatic cancer (and various other cancers) is that patients undergo a dramatic loss of body weight termed cancer associated cachexia. We have identified PTHrP as a potent cachexia-inducing factor in pancreatic cancer and are currently testing anti-PTHrP monoclonal neutralizing antibodies as a new therapeutic avenue to block or reverse cachexia. We are also leveraging our experience in patient-derived 3D organoid systems to find new tumor-derived cachexia-inducing factors.
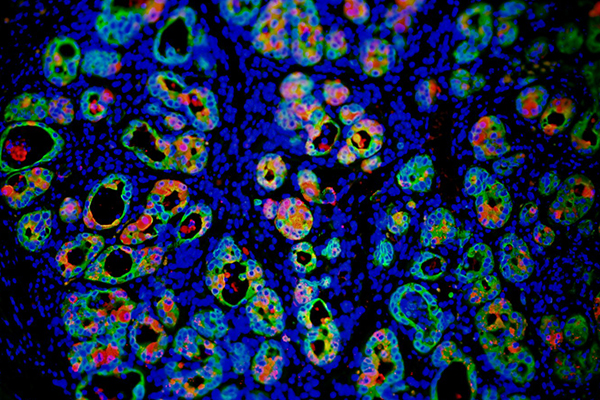
Epigenetic regulation of acinar-to-ductal metaplasia (ADM)
Pancreatic ductal adenocarcinoma (PDAC) was originally thought to be derived from resident pancreatic ductal cells. However, the prevailing theory is now that a different epithelial cell, acinar cells, are the cell of origin for PDAC. Acinar cells must undergo a requisite acinar-to-ductal metaplasia (ADM) to transdifferentiate into a ductal-like cell that eventually progresses to PDAC. We set out to explore the chromatin landscape as acinar cells undergo ADM, and we discovered new transcription factors that are able to unveil ductal lineage genes that are essential for this oncogenic transformation. In new mouse models, we have since deleted this transcription factor and have compelling evidence to suggest that it is essential for oncogenic ADM. We are now focusing on determining the role of this transcription factor in maintaining ductal cell identity as tumors evolve. We are designing screens to find new therapies to selectively target this transcription factor for destruction.
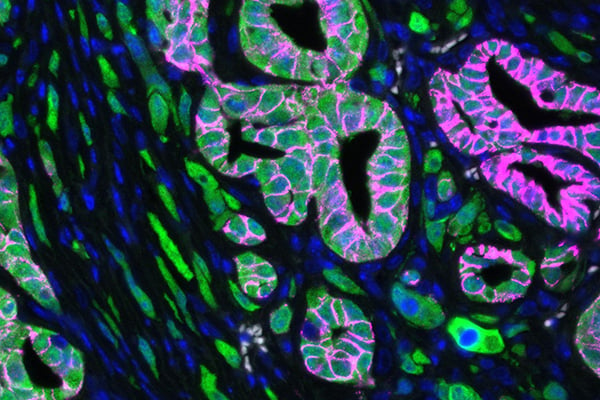
New subtype-specific mouse models of pancreatic cancer
It has recently become apparent that pancreatic cancer is comprised of various subtypes that are important for disease phenotypes. One such subtype, coined squamous (or quasi-mesenchymal/basal by other groups), is highly aggressive and nearly universally metastatic. Master regulators of this subtype have been identified in unbiased manner and we currently aim to build next-generation mouse models of pancreatic cancer that are subtype-specific to more faithfully mimic the patient’s disease.