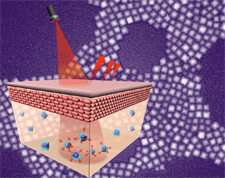
Neurobiologist Yang Xiang, PhD, assistant professor of neurobiology, finds evidence that neurons promote axon regeneration by communicating with nearby glia cells, according to a new paper in Developmental Cell.
“The dogma has always been that axon regeneration is controlled intrinsically from within a cell and central nervous system cells can’t regenerate,” said Dr. Xiang. “This study provides evidence that the external environment, in the form of glia cells, plays an important role in determining if an axon can regenerate or not. What’s more, when we flip this switch, we can turn on regeneration in neurons that normally can’t be repaired.”
The study shows that neurons in the peripheral nervous system (PNS) in Drosophila and central nervous system (CNS) in mice can be reprogrammed to regenerate. This discovery may be useful to scientists designing new treatments for a variety of disorders, from spinal cord injury to traumatic brain injury.
When mammalian neurons in the CNS are damaged from a traumatic injury, such as a car accident, or from neurodegenerative disorder, they typically can’t regenerate. What happens, according to Xiang, is that the long axon of the neuron, which is responsible for communication between the neuron and adjoining cells, is suspectable to damage and often becomes severed from the cell body. When this occurs, it is no longer able to function properly; many injured neurons can die.
Neurons from the PNS, such as the nerves found in the hand, can regenerate after injury. If an axon on a PNS neuron is damaged, the cell has the ability to regenerate and repair the damage. Neurons from the CNS, such as those found in the brain and spinal cord, don’t harbor this regenerative ability.
To distinguish why some neurons can regenerate, while others can’t, Xiang and colleagues designed an experiment with Drosophila larvae using powerful genetic tools to identify the mechanisms that control regeneration.
“The Drosophila larvae are unique in that they’re transparent and they allow us to observe axon injury and regeneration in intact animals,” said Fei Wang, PhD’23, former member of the Xiang lab, co-author of the study and current postdoctoral fellow at AbbVie Bioresearch Center in Worcester. “The fruit fly model also allows us to perform powerful genetics with the intention of finding underlying cellular and molecular programs that can potentially be used in mammalian neurons as well.”
Taking Drosophila larval neurons tuned to gentle touch sensation and comparing them to pain-sensing neurons, Xiang and colleagues identified an adenosine receptor active on the pain-sensing neurons that was absent from gentle touch neurons. When neurons are damaged, nearby glia cells increase their calcium signals, which causes glial to release adenosine. It’s this signaling, from glia to the adenosine receptor on the pain-sensing neuron, that stimulates its regeneration.
In the case of gentle touch neurons, however, Xiang found that glia were signaling for increased adenosine levels, but because the adenosine receptor wasn’t active, the neuron wasn’t able to receive the message necessary for regeneration. By genetically expressing the adenosine receptor on the gentle touch neuron, Xiang was able to turn on the cell’s regenerative program allowing it to communicate with the glia and regenerate its damaged axon.
This combination of gliotransmission and adenosine receptor activity drive axon regeneration in Drosophila, according to Xiang. Further studies in mouse models of damaged retinal ganglion cells showed that the same mechanism was at work in mammals. By activating the adenosine receptor on damaged retinal ganglion cells, Xiang showed that it’s possible to promote axon regeneration in CNS neurons in a mouse model of traumatic injury.
Together, this shows a shared role of the glia–neuron interaction for promoting axon regeneration between PNS and CNS neurons as well as between fly and mammalian animal models.
“The thinking had always been that glia prevented axon regeneration by forming a physical scar around the damaged neuron,” said Xiang. “Instead, it turns out that glia and gliotransmission are vital components in determining if an axon can regenerate after an injury.”
The next step for Xiang and colleagues is to investigate if gliotransmission can be used to develop novel strategies to promote recovery from spinal cord injury. Supporting this new research direction, Xiang and Zhigang He, PhD, professor of neurology at Boston Children’s Hospital and Harvard Medical School, received a $200,000 grant from the spinal cord research foundation Wings for Life.