Why Study Membrane Trafficking?
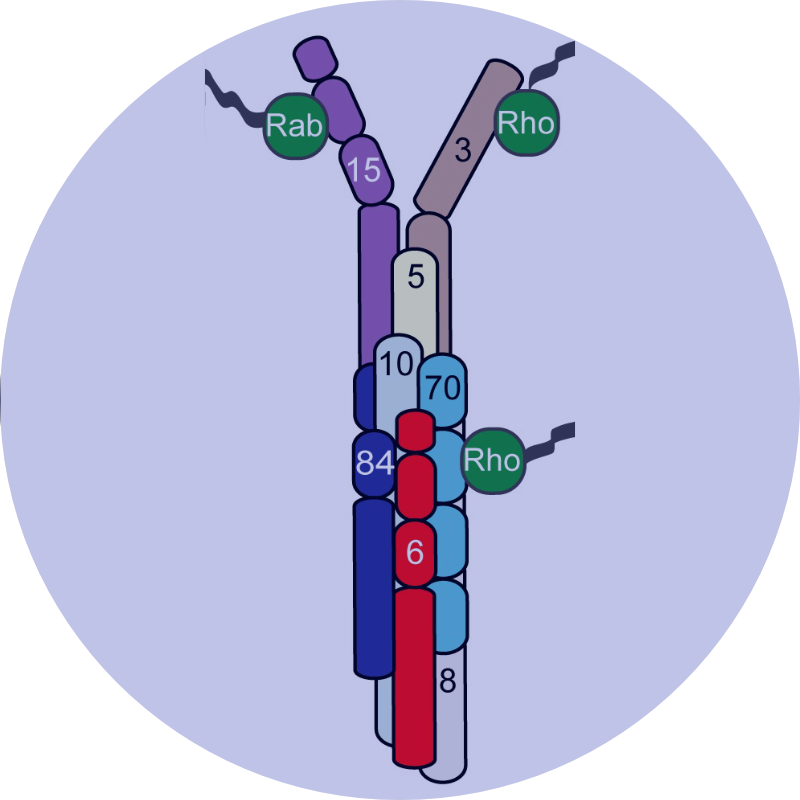
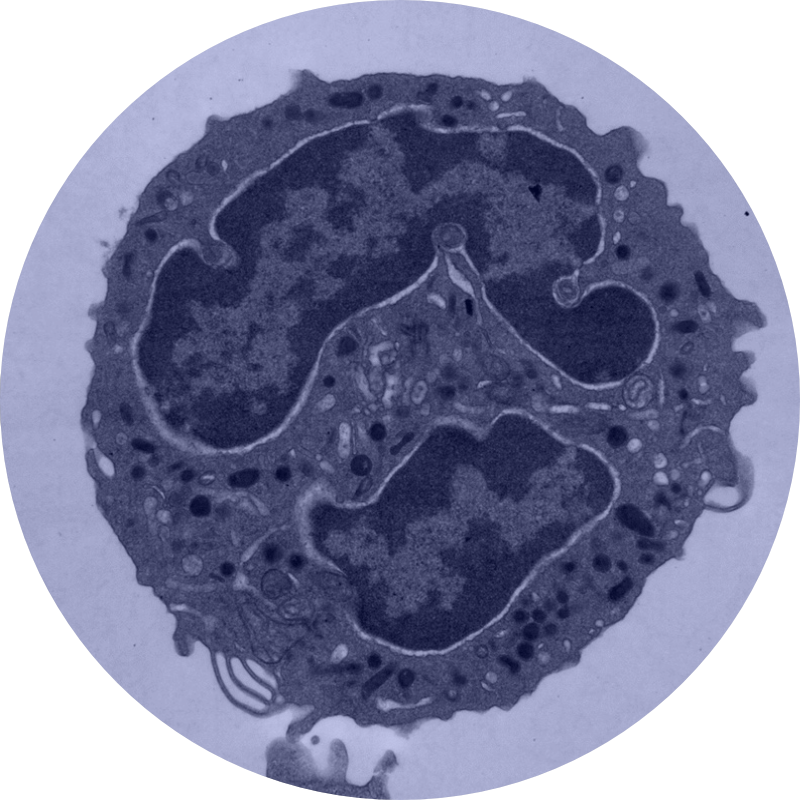
Our lab is interested in the regulation of membrane trafficking, in particular the mechanistic basis for the regulation of spatial and temporal specificity. Many questions remain to be answered regarding how membrane-bound vesicles containing specific cargo arrive at the correct location. For example, what marks the site of vesicle fusion on the target membrane and what checks to make sure that the correct vesicle docks at the right place? How are the membrane fusion proteins regulated to ensure that the wrong vesicle does not fuse? Our aim is to answer questions like these through a multifaceted approach that combines biochemistry, structural biology, cell biology, and biophysical techniques in a variety of model organisms.
x
Research Areas
Molecular illustrations on this webpage were generated by Leonora Martínez-Núñez, PhD.