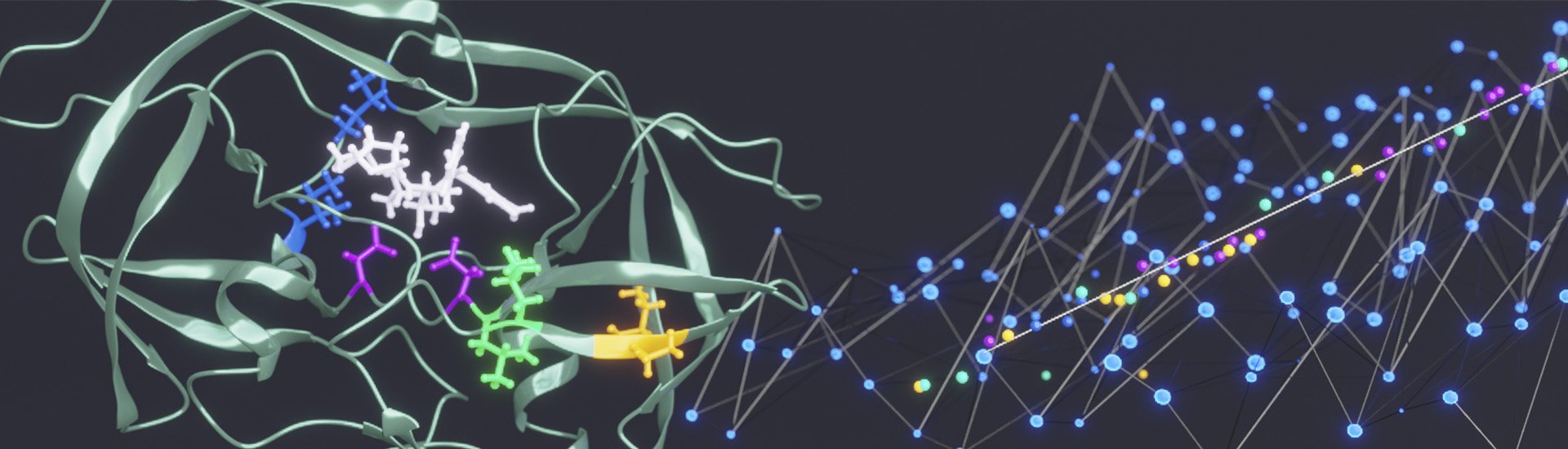
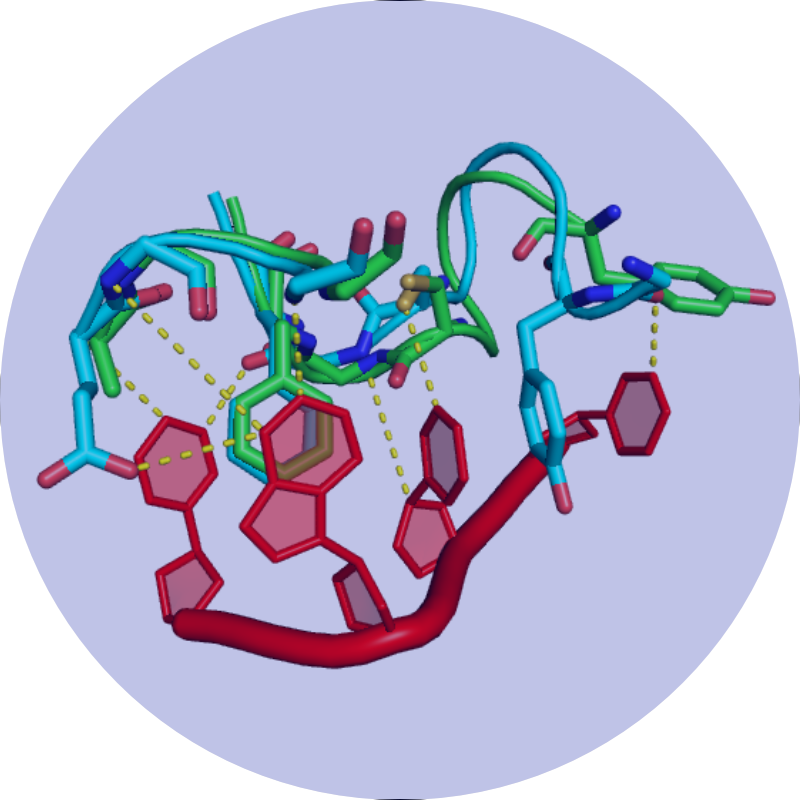
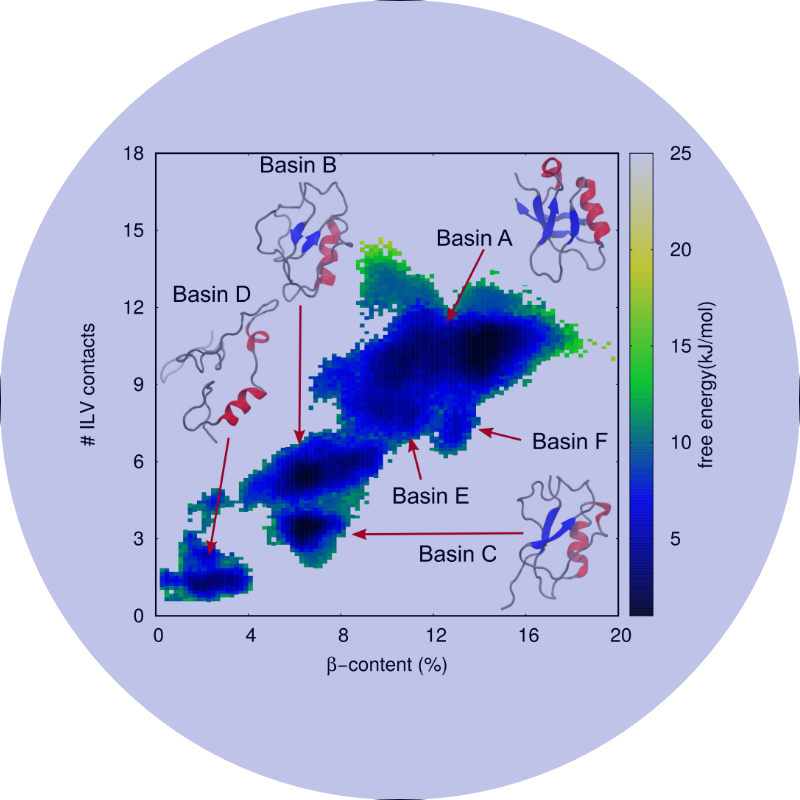
Our mission is to understand how conformational changes and internal dynamics affect protein function and mechanism of action.
x
The focus of my laboratory is to understand the relationship between the structure, stability, and dynamics of proteins. In particular we investigate how the structure and dynamics of a protein affect molecular recognition, binding specificity, allostery and stability. To this end, we take a multi-disciplinary approach combining the strengths of biophysical, biochemical and in vivo techniques, with particular emphasis on solution NMR spectroscopic and computational methods.
Research Areas
Commonly-Used Techniques
We utilize biophysical, biochemical, and in vivo techniques with a particular emphasis on NMR spectroscopy and computational methods.