Mechanisms of T cell Leukemogenesis
|
Although the survival rates have improved for children with T cell acute lymphoblastic leukemia (T-ALL), 25% of children relapse and most of these will eventually succumb to disease. Treatment of adults with T-ALL has been less successful, with long-term survival rates of only 10-30%. Moreover, modern dose-intensive chemotherapy is associated with side effects in survivors including organ damage/failure, neurocognitive deficits due to intrathecal prophylaxis and risk of developing secondary malignancies. The severity of these long-term side effects and rates of relapse urge the development of targeted therapies for this disease. We have modeled T-ALL in the mouse by expressing the TAL1 and LMO2 oncogenes in developing thymocytes. Similar to what is observed in cortical T-ALL patients, these mice develop spontaneous mutations in NOTCH1 and exhibit INK4A/ARF and/or PTEN loss (Figure 1). We were one of the first to demonstrate that mouse T-ALL is driven by rare leukemia-initiating cells (L-IC) and to show that the thymic progenitor DN3/4 population is enriched in its ability to initiate disease; whereas the CD4, CD8 double-positive leukemic cells were not (Figure 2). |
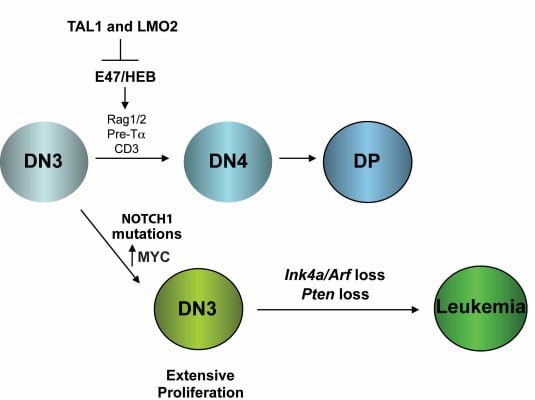 |
| Figure 1. Leukemia-initiating cells (L-IC) are a rare population of leukemic cells that are defined by their ability to initiate disease in mice. |
|
DN3 is enriched in L-IC activity Several studies suggest that relapse may result from a failure to eliminate leukemia-initiating cells (L-ICs). We have demonstrated that NOTCH1 inhibition reduces and in some cases eliminates mouse L-IC activity. Our work is innovative because it focuses on characterizing the recently identified DN3-enriched L-IC in our mouse T-ALL model and on determining the pathways that drive L-IC activity. We study L-IC biology in our mouse T-ALL model where hematopoietic development is well characterized and where micro environmental or niche influences on L-IC survival can be addressed. The current focus of the lab is to purify the mouse L-IC and to identify the pathways that mediate L-IC survival and self-renewal. |
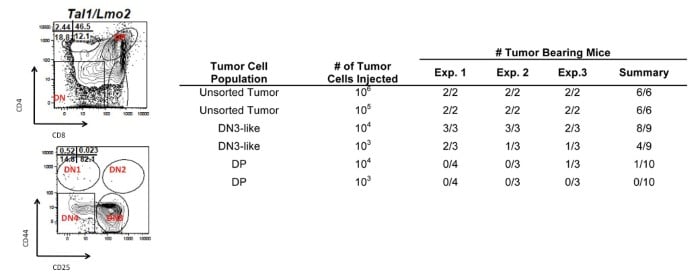 |
| Figure 2. Leukemic DN3 progenitors are enriched in leukemic-initiating potential. |
|
NOTCH/c-MYC pathway mediates L-IC activity NOTCH1 mutations develop spontaneously in our TAL1 and TAL1/LMO2 mouse T-ALL models and treatment with gamma-secretase inhibitors (GSI) prevents NOTCH1 activation and extends the survival of leukemic mice, demonstrating that GSIs have anti-leukemia activity in vivo (Cullion K, et. al. 2009). We have shown that NOTCH1 inhibition can also reduce/eliminate the L-IC population and prevent disease initiation (Tatarek J, et. al. 2011). NOTCH1 directly induces MYC expression (Sharma V, et. al. 2006) and MYC silencing or inhibition reduces mouse L-IC frequency (Figure 3; Roderick J, et. al. 2014). Collectively, these studies demonstrate that the NOTCH1-MYC axis is critical for L-IC activity in vivo. |
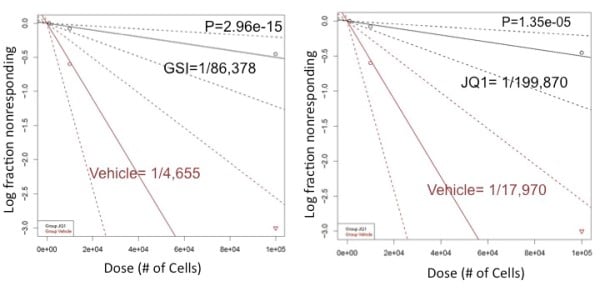 |
| Figure 3. Log-plot of L-IC frequency shows that NOTCH and MYC inhibition target the L-IC in Tal/Lmo2 T-ALL. |
References:
- Gutierrez A, Roderick JE, Kelliher MA. Leukemia propagating cells akt up. Cancer Cell. 2014 Mar 17; 25(3):263-5.
- Roderick JE, Tesell J, Shultz LD, Brehm MA, Greiner DL, Harris MH, Silverman LB, Sallan SE, Gutierrez A, Look AT, Qi J, Bradner JE, Kelliher MA. c-Myc inhibition prevents leukemia initiation in mice and impairs the growth of relapsed and induction failure pediatric T-ALL cells. Blood. 2014 Feb 13; 123(7):1040-50.
- Mansour MR, Sanda T, Lawton LN, Li X, Kreslavsky T, Novina CD, Brand M, Gutierrez A, Kelliher MA, Jamieson CH, von Boehmer H, Young RA, Look AT. The TAL1 complex targets the FBXW7 tumor suppressor by activating miR-223 in human T cell acute lymphoblastic leukemia. J Exp Med. 2013 Jul 29;210(8):1545-57.
- Tatarek J, Cullion K, Ashworth T, Gerstein R, Aster JC, Kelliher MA. Notch1 inhibition targets the leukemia-initiating cells in a Tal1/Lmo2 mouse model of T-ALL. Blood. 2011 Aug 11; 118(6):1579-90.
- Draheim KM, Hermance N, Yang Y, Arous E, Calvo J, Kelliher MA. A DNA-binding mutant of TAL1 cooperates with LMO2 to cause T cell leukemia in mice. Oncogene. 2011 Mar 10; 30(10):1252-60.
- Cullion K, Draheim KM, Hermance N, Tammam J, Sharma VM, Ware C, Nikov G, Krishnamoorthy V, Majumder PK, Kelliher MA. Targeting the Notch1 and mTOR pathways in a mouse T-ALL model. Blood. 2009 Jun 11; 113(24):6172-81.
- Sharma VM, Draheim KM, Kelliher MA. The Notch1/c-Myc pathway in T cell leukemia. Cell Cycle. 2007 Apr 15; 6(8):927-30.
- Sharma VM, Calvo JA, Draheim KM, Cunningham LA, Hermance N, Beverly L, Krishnamoorthy V, Bhasin M, Capobianco AJ, Kelliher MA. Notch1 contributes to mouse T-cell leukemia by directly inducing the expression of c-myc. Mol Cell Biol. 2006 Nov; 26(21):8022-31.
- Chang PY, Draheim K, Kelliher MA, Miyamoto S. NFKB1 is a direct target of the TAL1 oncoprotein in human T leukemia cells. Cancer Res. 2006 Jun 15; 66(12):6008-13.
- Shank-Calvo JA, Draheim K, Bhasin M, Kelliher MA. p16Ink4a or p19Arf loss contributes to Tal1-induced leukemogenesis in mice. Oncogene. 2006 May 18; 25(21):3023-31.
- O'Neil J, Calvo J, McKenna K, Krishnamoorthy V, Aster JC, Bassing CH, Alt FW, Kelliher M, Look AT. Activating Notch1 mutations in mouse models of T-ALL. Blood. 2006 Jan 15; 107(2):781-5.
- O'Neil J, Shank J, Cusson N, Murre C, Kelliher M. TAL1/SCL induces leukemia by inhibiting the transcriptional activity of E47/HEB. Cancer Cell. 2004 Jun; 5(6):587-96.
- O'Neil J, Ventura JJ, Cusson N, Kelliher M. NF-kappaB activation in premalignant mouse tal-1/scl thymocytes and tumors. Blood. 2003 Oct 1; 102(7):2593-6.
- O'Neil J, Billa M, Oikemus S, Kelliher M. The DNA binding activity of TAL-1 is not required to induce leukemia/lymphoma in mice. Oncogene. 2001 Jun 28; 20(29):3897-905.
