RIP Kinases in Inflammation and Innate Immune Signaling
|
RIP kinase 1 (RIPK1) is the founding member of a serine-threonine kinase family that transduces inflammatory and cell death signals following death receptor ligation, activation of pattern recognition receptors (PRRs) and DNA damage. RIPK1 is the core component of TNF-induced signaling complexes mediating NFκB and MAP kinase activation, apoptosis and necroptosis, which has recently emerged as a prominent cellular defense mechanism against viral infection (Figure 1). Although not required for TNF-induced NFκB or MAP kinase activation, the kinase activities of RIPK1 and the related RIPK3 are required for necroptosis. Our understanding of RIPK1 has been hindered by an inability to carry out in vivo studies due to the neonatal lethality associated with a complete RIPK1 deficiency. We generated a Ripk1-deficient mouse and demonstrated that a RIPK1 deficiency results in sensitivity to TNF-induced cell death which is partially rescued by the absence of TNF receptor (Tnfr1) (Kelliher M, et. al. 1998; Cusson N, et. al. 2002). These studies revealed that RIPK1 provides a critical survival signal. To identify tissues and cell types dependent on RIPK1 for survival we developed conditional Ripk1 mice and kinase inactive RIPK1 D138N mice (Figure 2), enabling us to pursue the involvement of RIPK1 in the anti-viral innate immune response and necroptosis rigorously. The knowledge gained from these in vivo studies is essential if we are to use RIPK1 kinase specific inhibitors to limit the inflammatory response to tissue injury without compromising innate immune defenses. |
 |
|
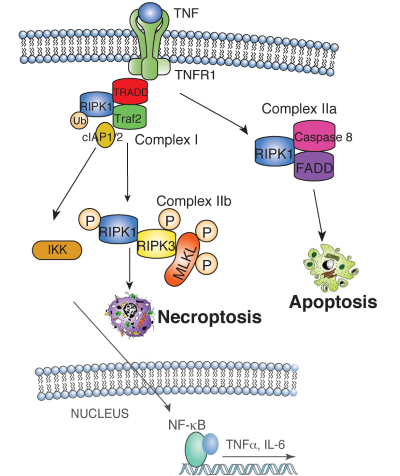
| Figure 1. TNF binding to the TNF receptor 1 (TNFR1) stimulates the recruitment of TRADD, which recruits RIPK1 to stimulate NFkB or MAP kinase activation, leading to cytokine and chemokine production. RIPK1 is ubiquitin-modified by the IAP1/2/TRAF2 complex, which recruits and activates the heterotrimeric IKK complex. When RIPK1 ubiquitination is inhibited, RIPK1 binds Caspase 8 and FADD to induce apoptosis. Under conditions when Caspase 8 activity is inhibited, RIPK1 phosphorylates RIPK3, which then phosphorylates MLKL, resulting in MLKL translocation to the plasma membrane. MLKL translocation results in changes in membrane permeability, DAMP release and induction of necroptosis. | ||
|
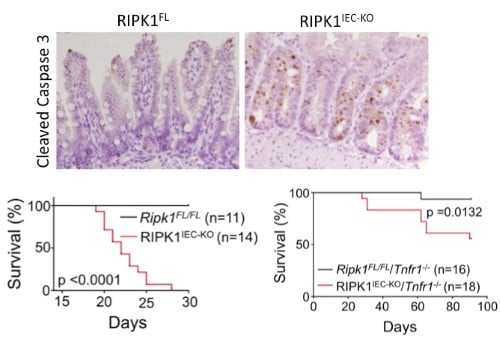
RIPK1 maintains epithelial homoestasis RIPK1 is implicated in inflammatory and cell death signaling and its kinase activity is thought to drive RIPK3-mediated necroptosis. Studies in the lab have shown that RIPK1 has kinase-independent scaffolding functions that regulate homeostasis and prevent inflammation in barrier tissues by inhibiting epithelial cell apoptosis and necroptosis (Dannappel M, et.al. 2014). Intestinal epithelial cell (IEC) specific deletion of RIPK1 caused IEC apoptosis, villus atrophy, loss of goblet and Paneth cells and premature death in mice that was partially rescued by a TNFR1 deficiency (Figure 2). Epidermis-specific RIPK1 deletion triggered keratinocyte apoptosis and necroptosis and caused skin inflammation that was prevented by RIPK3 deficiency. A RIPK1 kinase inactive knock-in (Figure 4) delayed but did not prevent inflammation. These studies reveal that RIPK1 inhibits RIPK3-mediated necroptosis in vivo. |
||
 |
||
| Figure 2. Intestinal epithelial cell (IEC) specific knockout of RIPK1 causes IEC apoptosis, villus atrophy, loss of goblet and Paneth cells and premature death of the mice. Tnfr1 deficiency prolongs survival and ameliorates intestinal pathology of RIPK1IEC-KO mice. | ||
|
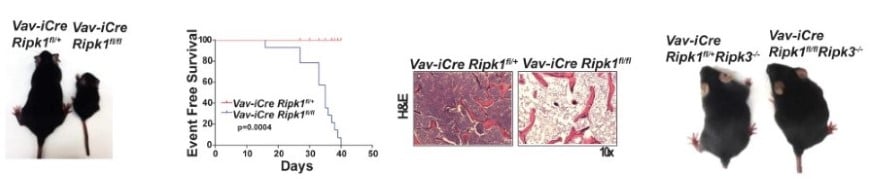
Hematopoietic RIPK1 deficiency results in bone marrow failure To identify the lineages and cell types that depend on RIPK1 for survival, we generated conditional Ripk1 mice and examined the effects of Ripk1 deletion in the hematopoietic lineage. A hematopietic RIPK1 deficiency stimulates pro-inflammatory cytokine and chemokine production and hematopoietic cell death, resulting in bone marrow failure. We demonstrate that a RIPK3 deficiency partially rescues hematopoiesis, revealing that RIPK1 mediates survival by preventing RIPK3-mediated necroptosis (Figure 3). These data reveal anti-inflammatory roles for RIPK1, suggesting that RIPK1 negatively regulates RIPK3 activation. Our studies may have translational implications as human bone marrow syndromes such as Aplastic Anemia and Fanconi Anemia overproduce and are hypersensitive to TNF and γ-IFN. |
||
 |
||
| Figure 3. Deletion of RIPK1 in the hematopoietic lineage results in acute bone marrow failure that is rescued by RIPK3 deficiency. | ||
|
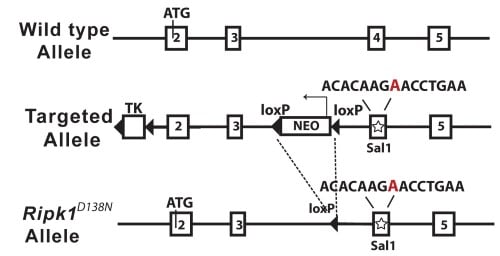
Generation of kinase inactive RIPK1 D138N mice |
||
|
|
||
| Figure 4. Schematic diagram of the mouse Ripk1 locus and the RIPK1D138N allele | ||
|
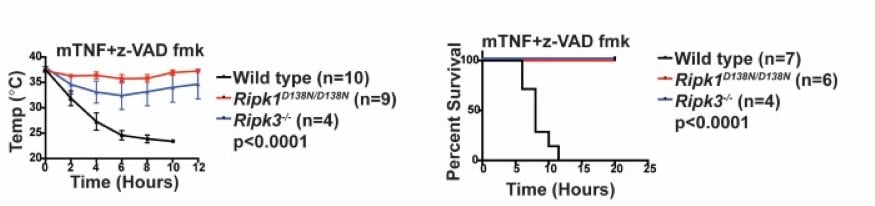
RIPK1 kinase inactive mice are viable and protected from TNF-induced necroptosis To determine the contribution of RIPK1 kinase to inflammation in vivo, we generated knock-in mice endogenously expressing catalytically inactive RIPK1 D138N. Unlike Ripk1 -/- mice, which die shortly after birth, RIPK1 D138N/D138N mice are viable. Cells expressing RIPK1 D138N are resistant to TNF- and poly (I:C)-induced necroptosis in vitro and remarkably RIPK1 D138N/D138Nmice are protected from TNF-induced shock in vivo (Figure 5). |
||
 |
||
| Figure 5. RIPK1D138N mice are protected from TNF-induced shock. | ||
References:
- Roderick JE, Hermance N, Zelic M, Simmons MJ, Polykratis A, Pasparakis M, Kelliher MA. Hematopoietic RIPK1 deficiency results in bone marrow failure caused by apoptosis and RIPK3-mediated necroptosis. Proc Natl Acad Sci U S A. 2014 Oct 7;111(40):14436-41.
- Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T, Hermance N, Zelic M, Kirsch P, Basic M, Bleich A, Kelliher M, Pasparakis M. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014 Sep 4;513(7516):90-4. doi: 10.1038/nature13608.
- Polykratis A, Hermance N, Zelic M, Roderick J, Kim C, My Van T, Lee T, Chan F, Pasparakis M, Kelliher M. Cutting Edge: RIPK1 Kinase Inactive Mice Are Viable and Protected from TNF-Induced Necroptosis In Vivo. J Immunol. 2014
- Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, Janke LJ, Kelliher MA, Kanneganti TD, Green DR. RIPK1 Blocks Early Postnatal Lethality Mediated by Caspase-8 and RIPK3. Cell. 2014 May 22; 157(5):1189-202.
- Yang Y, Xia F, Hermance N, Mabb A, Simonson S, Morrissey S, Gandhi P, Munson M, Miyamoto S, Kelliher MA. A cytosolic ATM/NEMO/RIP1 complex recruits TAK1 to mediate the NF-kappaB and p38 mitogen-activated protein kinase (MAPK)/MAPK-activated protein 2 responses to DNA damage. Mol Cell Biol. 2011 Jul; 31(14):2774-86.
- Coulombe F, Divangahi M, Veyrier F, de Leseleuc L, Gleason JL, Yang Y, Kelliher MA, Pandey AK, Sassetti CM, Reed MB, Behr MA. Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J Exp Med. 2009 Aug 3; 206(8):1709-16.
- Pandey AK, Yang Y, Jiang Z, Fortune SM, Coulombe F, Behr MA, Fitzgerald KA, Sassetti CM, Kelliher MA. NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog. 2009 Jul; 5(7):e1000500.
- Yang Y, Yin C, Pandey A, Abbott D, Sassetti C, Kelliher MA. NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2. J Biol Chem. 2007 Dec 14; 282(50):36223-9.
- Abbott DW, Yang Y, Hutti JE, Madhavarapu S, Kelliher MA, Cantley LC. Coordinated regulation of Toll-like receptor and NOD2 signaling by K63-linked polyubiquitin chains. Mol Cell Biol. 2007 Sep; 27(17):6012-25.
- Huye LE, Ning S, Kelliher M, Pagano JS. Interferon regulatory factor 7 is activated by a viral oncoprotein through RIP-dependent ubiquitination. Mol Cell Biol. 2007 Apr; 27(8):2910-8.
- Cusson-Hermance N, Khurana S, Lee TH, Fitzgerald KA, Kelliher MA. Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-{kappa}B activation but does not contribute to interferon regulatory factor 3 activation. J Biol Chem. 2005 Nov 4; 280(44):36560-6.
- Lee TH, Shank J, Cusson N, Kelliher MA. The kinase activity of Rip1 is not required for tumor necrosis factor-alpha-induced IkappaB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J Biol Chem. 2004 Aug 6; 279(32):33185-91.
- Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004 May; 5(5):503-7.
- Lee TH, Huang Q, Oikemus S, Shank J, Ventura JJ, Cusson N, Vaillancourt RR, Su B, Davis RJ, Kelliher MA. The death domain kinase RIP1 is essential for tumor necrosis factor alpha signaling to p38 mitogen-activated protein kinase. Mol Cell Biol. 2003 Nov; 23(22):8377-85.
- Cusson N, Oikemus S, Kilpatrick ED, Cunningham L, Kelliher M. The death domain kinase RIP protects thymocytes from tumor necrosis factor receptor type 2-induced cell death. J Exp Med. 2002 Jul 1; 196(1):15-26.
- Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998 Mar; 8(3):297-303.