I’ve recently received many requests for an update on the new ruxolitinib cream to treat vitiligo. To remind everyone, we initially reported that oral ruxolitinib, a Janus Kinase inhibitor initially approved to treat a type of blood cancer, reversed disease in a patient with vitiligo (read a blog about this here). Next, a study was done in 12 patients testing this same drug but as a topical cream for vitiligo, which also showed improvement (read about this here). Then, the company that makes the oral drug for the blood cancer, Incyte, decided to conduct a Phase 2 clinical trial in over 150 patients in the US to see if this topical might be effective for vitiligo. This was a placebo-controlled, double-blinded, multicenter clinical trial, an approach that provides the best evidence about whether a medicine works or not.
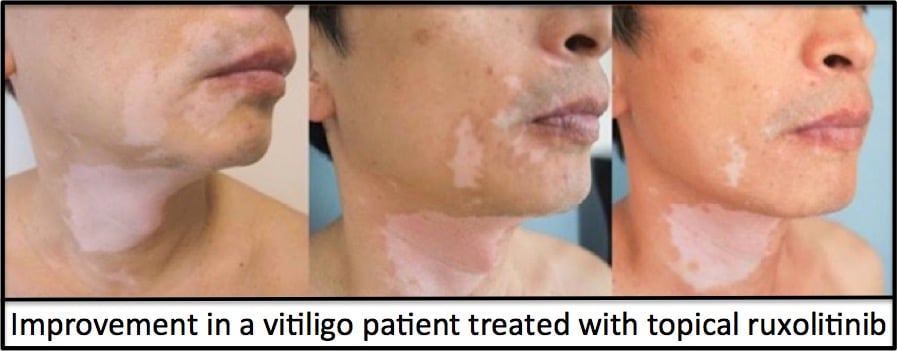
It turns out that the Phase 2 clinical trial showed very promising results at the 6-month time point (read about that here) and excellent results at the end of 1 year of treatment (read about that here). Below is a picture of the results after 1 year of treatment:
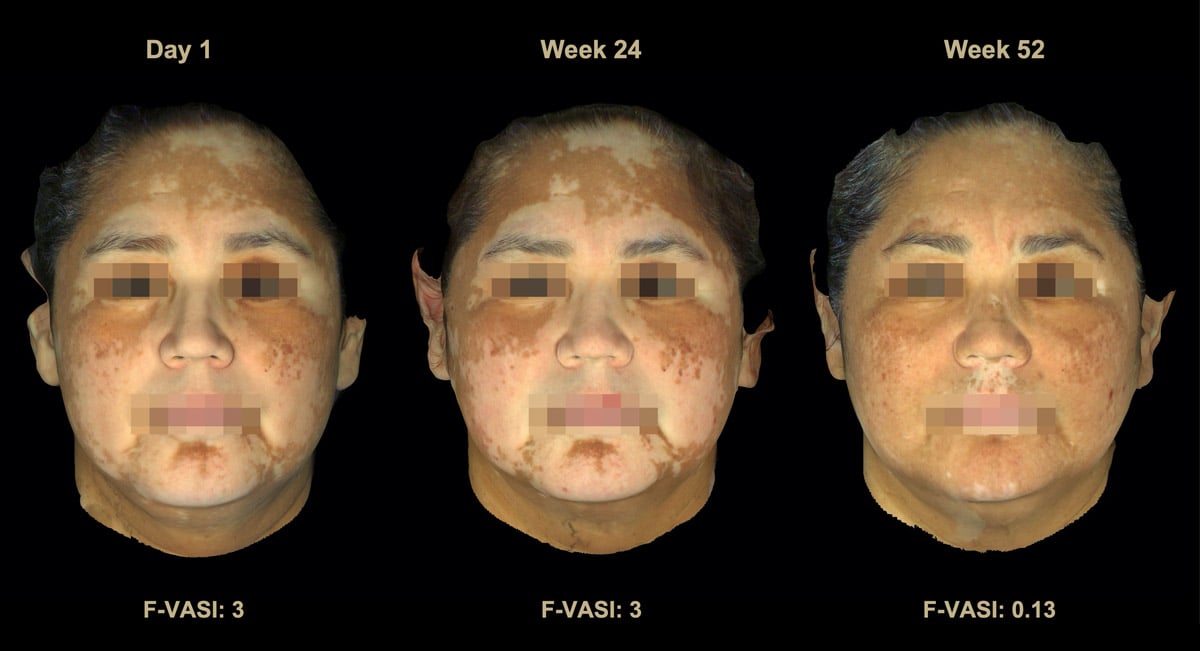
Not only were the results very good, but it appeared to be a very safe treatment, as the only side effect reported more commonly in those using the drug vs. the placebo was acne appearing in the location of the treatment, which occurred in about 10-15% of users. We published the results of this trial in The Lancet, one of the most prestigious scientific medical journals. We recently announced 2-year results from this study, which revealed that even more patients who used the cream achieved 50-75-90% improvement in their disease after an additional year of treatment. In fact, another 30% of those in the trial achieved these key milestones after an extra year of treatment (read the results).
Based on this data, Incyte decided to run a Phase 3 Clinical Trial in over 600 patients all over the world, with the goal of supporting an application to the FDA (and the European equivalent, the EMA) to approve the treatment for patients. This means it would be available for prescription from the pharmacy, and should be covered by health insurance companies, as it would become the first and only FDA-approved treatment to reverse the effects of vitiligo! Well, we recently heard (although the data isn’t publicly available yet) that the results of the Phase 3 trial were excellent (see the announcement)! In fact, the Phase 3 results looked very similar to the Phase 2 results (a very good sign that the treatment works as hoped), and there were no additional safety concerns, suggesting that we have a winner! Because the Phase 3 study included children aged 12 years and older, the FDA approval will likely include this age range, making the new treatment available for children as well.
The next step is to submit the application to the FDA for final approval of topical ruxolitinib for the treatment of vitiligo. They can take up to 1 year to review the documents before approval, but they already are reviewing this same treatment for another disease, so maybe things will move faster. We’re hoping for approval by early next year, keep your eyes open for more updates!