
When her high school biology teacher drew the metabolic pathway for glycolysis on the board, Jessica Spinelli, PhD, was mesmerized. Describing it as one of her “nerdiest moments ever,” she credits the incident with helping cement her decision to become a scientist. Starting at UMass Chan in July 2022 as an assistant professor in the Program in Molecular Medicine, Dr. Spinelli leads a team of seven, and revels in watching them build confidence as they begin to make independent discoveries.
With two first-author Science papers already under her belt and a host of fresh ideas to tackle, her career is off to a roaring start. Propelled by her ingenuity, she is poised to help us understand the inner mechanics of metabolism, including how mitochondria—the cell’s battery—seamlessly adapt to oxygen-deprived environments to provide the sustenance tumor cells need for survival.
You highlight a discrete moment when you were “hooked on” science. Did you immediately know you would study metabolism for a living, or was it more gradual?
That iconic moment in high school biology pointed me toward majoring in biochemistry in college, but it didn’t outline a precise path. It took a while before I started doing what I love—metabolism research. First, I worked part-time in a microbiology laboratory as part of a fellowship the Howard Hughes Medical Institute provided. And, although that job was better than folding clothes in the mall, the topic didn't compel me. Then, when I went to graduate school, again I didn’t immediately join a metabolism lab—but this time, it was my fault. I decided to rotate through different labs first. As was the case with the microbiology job, the topics didn’t grab me. When I finally started in a metabolism lab, I was immediately sucked in. I should have listened to my heart at the start!
Much of your research focuses on the electron transport chain (ETC) that operates in the cell’s mitochondria. Can you introduce this concept?
The ETC is a set of protein complexes that transport electrons inside the mitochondria. The objective is to pump protons through the chain as electrons are transferred in each protein complex, helping support many key mitochondrial functions including synthesis of adenosine triphosphate or ATP. Oxygen is the final electron acceptor in this chain and its consumption is needed to maintain this electron flow in the ETC. Some of my research focuses on how mitochondrial functions, like ATP synthesis, are achieved when oxygen can’t be used as an electron acceptor—as in cancer cells.
Why is the electron transport chain important in cancer?
We know that cancer cells can’t survive without an active ETC. We also know that the ETC typically needs oxygen to function, which is a problem because tumor cells are low in oxygen. So, we suspected that cancer cells must adapt to an oxygen-deprived environment, preserving the ETC.
Before I came to UMass Chan, my colleagues and I asked whether another electron acceptor assumed oxygen's role, and if so, which one? We knew that other organisms, like bacteria, use fumarate, sulfates or nitrates as electron acceptors. So, we experimented with those chemicals, finding that tumor cells use a small molecule metabolite called fumarate as its electron acceptor, helping their mitochondria survive without oxygen.
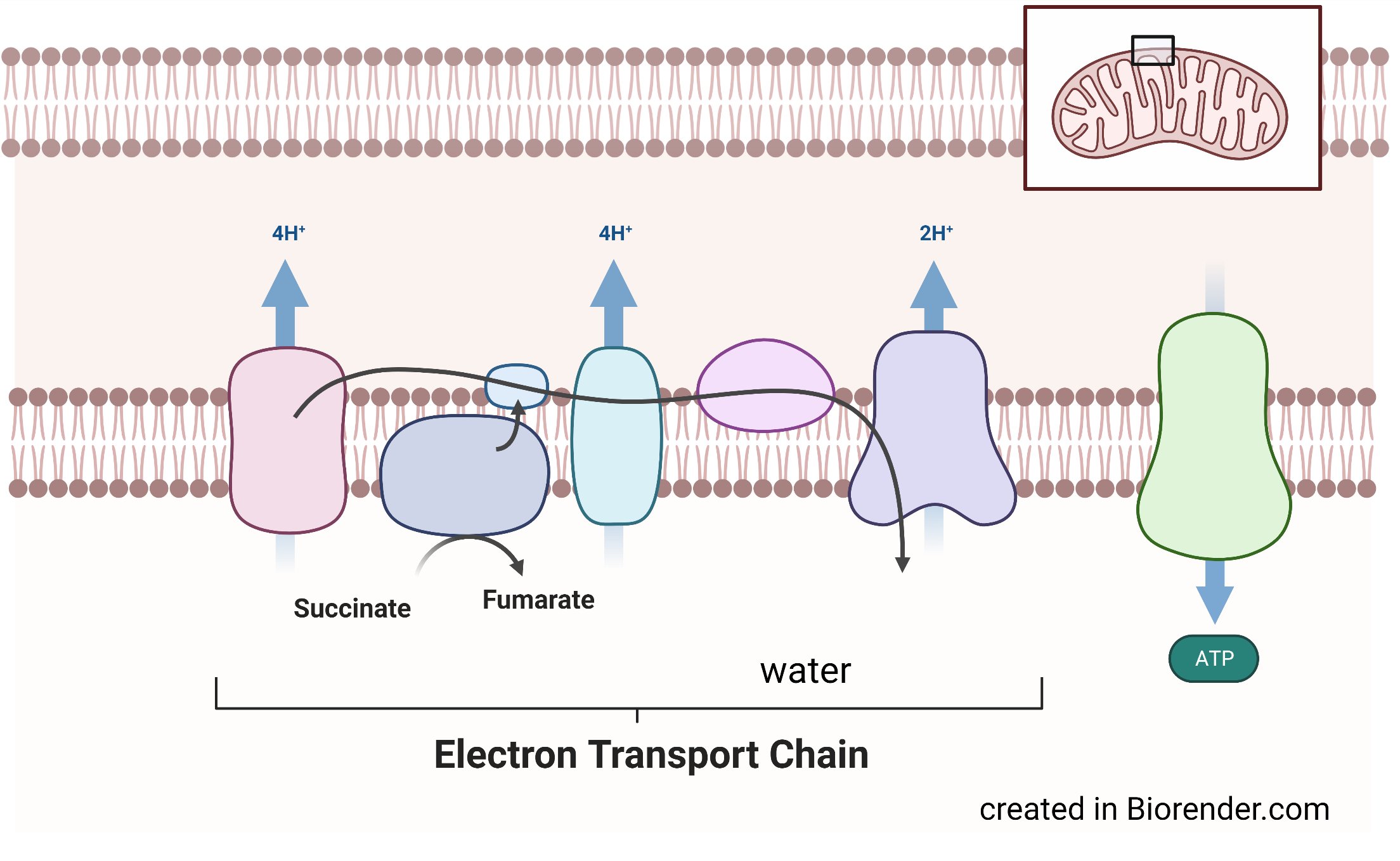
What’s particularly exciting is that I don't think we’ve finished identifying all the other electron acceptors that can support mitochondrial function in mammals. I think we've only started to scratch the surface!
This finding landed you a Science article. When did you know that you were onto something of great significance?
When I saw that the biosynthesis of essential compounds like pyrimidines—one of the compounds used to make the building blocks of DNA and RNA—could still take place with fumarate as the final electron acceptor, I knew we discovered something that applied more broadly to mammalian biology and not just to cancer biology.
What tools did you use to make this discovery?
We use stable isotope tracing combined with high resolution mass spectrometry to track the use of fumarate as an electron acceptor. Isotopes are two or more forms of the same element (e.g., carbon) that differ only in their number of neutrons, giving each a different atomic mass. Mass spectrometry is a tool that measures the atomic weights of molecules based on mass-to-charge ratios.
To determine whether fumarate could be used as an electron acceptor, we labeled fumarate with 13C, which is a less common isotope of carbon than 12C. Then, we used the 13C as a barcode to track the journey fumarate took, providing information about its chemical breakdown, the metabolic pathways it entered, and the chemical processes that took place—such as its conversion into the chemical succinate, which occurs when fumarate is used as an electron acceptor. We then used mass spectrometry to distinguish the fumarate isotopes from other metabolites of interest to help us draw our conclusions.
Where do you think the field of cancer metabolism research is heading?
People are gaining interest in:
1) how dietary interventions–such as the ketogenic diet—impact tumor growth,
2) understanding how the tissue of origin, such as pancreas or lung, influences tumor metabolism. For example, pancreatic and lung cancer use different adaptation strategies to nourish the cancer cells. We need a better conception of the properties driving these dissimilarities to identify metabolic vulnerabilities and potential therapeutic targets in different tumor subtypes.
3) the interactions between different cell types in the tumor microenvironment and how they influence tumor growth.
What is the most enjoyable part of your job as a researcher?
I love mentoring my trainees, watching them solve problems, gain confidence, and be bold enough to generate their own ideas. I even open my lab to students in high school because I think providing the opportunity to learn science to this young age group—without watering it down or sugar-coating it—gives students an ideal opportunity to find their passion for research.
About Jessica Spinelli, PhD
Jessica Spinelli, PhD, is an assistant professor in the Program of Molecular Medicine at the University of Massachusetts Chan Medical School. She earned her PhD in Chemical Biology at Harvard University. She serves as co-organizer of the UMass Metabolic Network monthly seminar series.