Wednesday, April 07, 2021
|
Oropharyngeal cancer is a type of head and neck cancer occurring in the oropharynx—the region of the throat that includes the tonsils, soft palate and base of the tongue. The vast majority of oropharyngeal cancers arise in the cells lining these tissues, called squamous cells, and are known as oropharyngeal squamous cell carcinoma (OPSCC).
About 70% of OPSCCs are caused by human papillomavirus (HPV). In the United States, the rate of HPV-positive OPSCC is increasing, especially among middle-aged adults. The disease is asymptomatic in the early stages, and is therefore often diagnosed at an advanced stage in which the cancer has spread to lymph nodes or surrounding tissue. In general, HPV-positive OPSCC has a favorable prognosis compared to HPV-negative disease, however about 20-25% of HPV-positive OPSCC patients develop recurrent cancer.
Study At A Glance
A Phase II/III Randomized Study of Maintenance Nivolumab Versus Observation in Patients With Locally Advanced, Intermediate Risk HPV-Positive OPSCC.
This trial randomizes patients with advanced HPV-positive OPSCC to undergo concurrent definitive therapy—chemotherapy (cisplatin) plus intensity-modulated radiotherapy—followed by either maintenance immunotherapy (nivolumab) or no treatment (observation). Patients are then monitored for progression-free survival (phase II) and overall survival (phase III).
Although the use of immunotherapy has been shown to improve outcomes for people with advanced HPV-positive OPSCC, it is not yet known whether adding immunotherapy as a maintenance treatment can help prevent the disease from returning in patients whose cancer has been cured by chemotherapy and radiation. The goal of this trial is to determine if adding maintenance immunotherapy as part of the treatment regimen can improve patient outcomes compared to chemotherapy and radiation therapy alone.
Study Overview
This clinical trial offers treatment to patients who have stage 2 or stage 3 HPV-positive OPSCC that has spread to nearby lymph nodes or tissue, such as the epiglottis, larynx or jaw. Patients first receive a combination of two standard therapies: (1) a chemotherapeutic called cisplatin, which is a platinum-based drug that kills cancer cells by causing DNA damage, and (2) an advanced type of radiation therapy called intensity-modulated radiotherapy (IMRT), which uses computer programs to calculate and deliver a precise radiation dose based on the shape of the tumor. These two treatments are given at the same time (called concurrent therapy) for a period of 7 weeks. The treatment is designed to cure the cancer (known as definitive therapy).
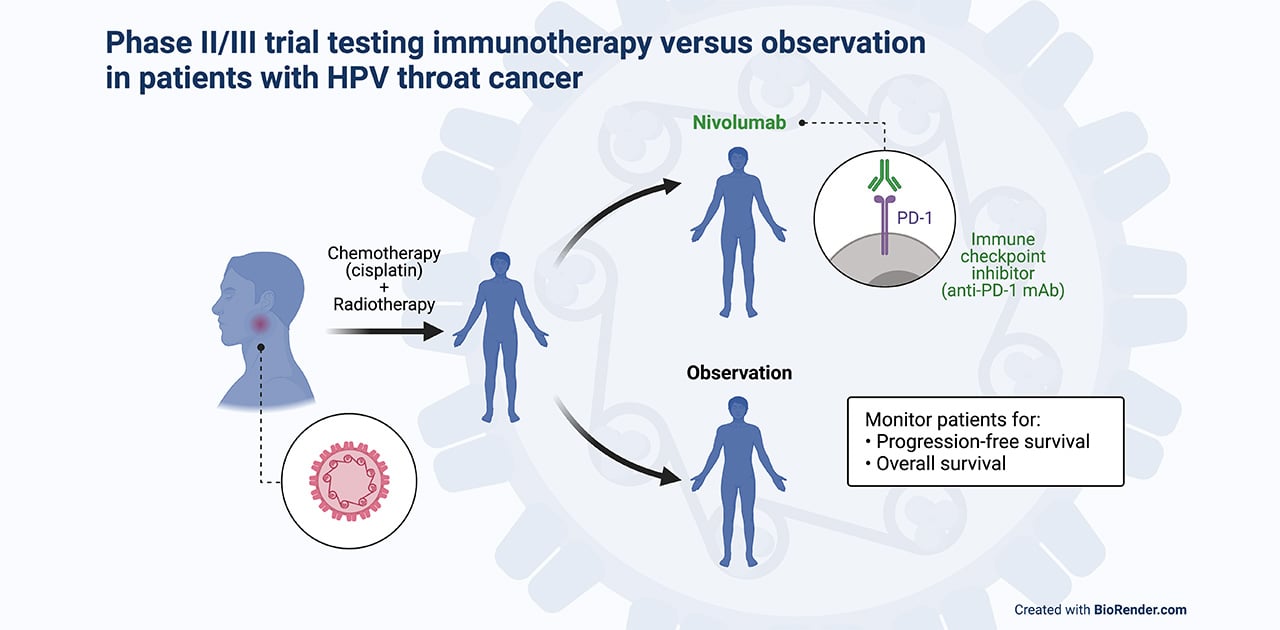
Within 4 weeks of completing the concurrent definitive therapy, patients randomly receive either maintenance immunotherapy for 12 months or no further treatment. The immunotherapy drug is the immune checkpoint inhibitor nivolumab, a targeted monoclonal antibody that binds to PD-1, a receptor protein present on the surface of immune cells (T cells), and blocks it from interacting with its ligand, PD-L1, a protein present on the tumor cells. The interaction between PD-1 and PD-L1 prevents the immune cell from destroying the cancer cell; blocking this interaction using nivolumab restores the ability of the T cells to kill the tumor cell. In this way, nivolumab is designed to help the body's immune system attack the cancer if it returns.
Both groups of patients in the study will be monitored long-term, up to 10 years. If patients who have not received immunotherapy have their cancer return within a year from the end of concurrent definitive therapy, they will be offered the option of receiving nivolumab. The study will compare those who have received chemotherapy/radiotherapy followed by nivolumab to those who have received chemotherapy/radiotherapy followed by observation to determine whether maintenance immunotherapy results in a significant improvement in progression-free survival and overall survival outcomes.
The National Cancer Institute-sponsored clinical trial hopes to enroll about 750 participants nationwide. UMass Chan is one of ~600 study locations in the country, and one of two locations in Massachusetts, offering the trial. At UMass, the study is being led by Dr. William Walsh, MD, an associate professor in the Department of Medicine, Division of Hematology-Oncology, and Medical Director of the Cancer Research Office at UMass Chan Medical School.
How to Enroll?