
Discovering new treatments for myeloma has been a priority for UMass Cancer Center researcher Dr. Muthalagu Ramanathan, MD, who is an associate professor in the Division of Hematology-Oncology, Co-Director of the Blood and Bone Marrow Transplant Program and Director of the Myeloma Program at UMass Chan Medical School and UMass Memorial Health Care. Her research focus is in offering national myeloma clinical trials to patients at UMass. She is currently leading a trial at UMass that explores the effectiveness of different maintenance treatments following autologous stem cell transplant (ASCT).
Study At A Glance
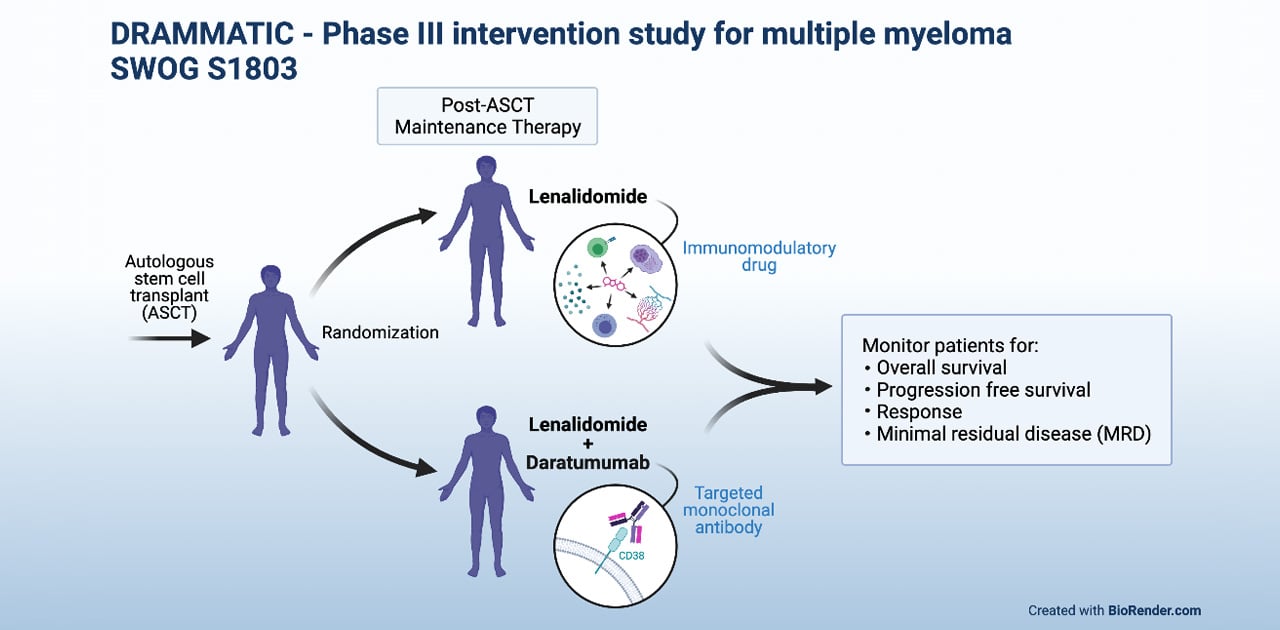
SWOG S1803 – Phase III Study of Daratumumab (NSC- 791647) + Lenalidomide (LD) or Lenalidomide (L) as Post-Autologous Stem Cell Transplant Maintenance Therapy in Patients with Multiple Myeloma (MM) Using Minimal Residual Disease to Direct Therapy Duration (DRAMMATIC Study).
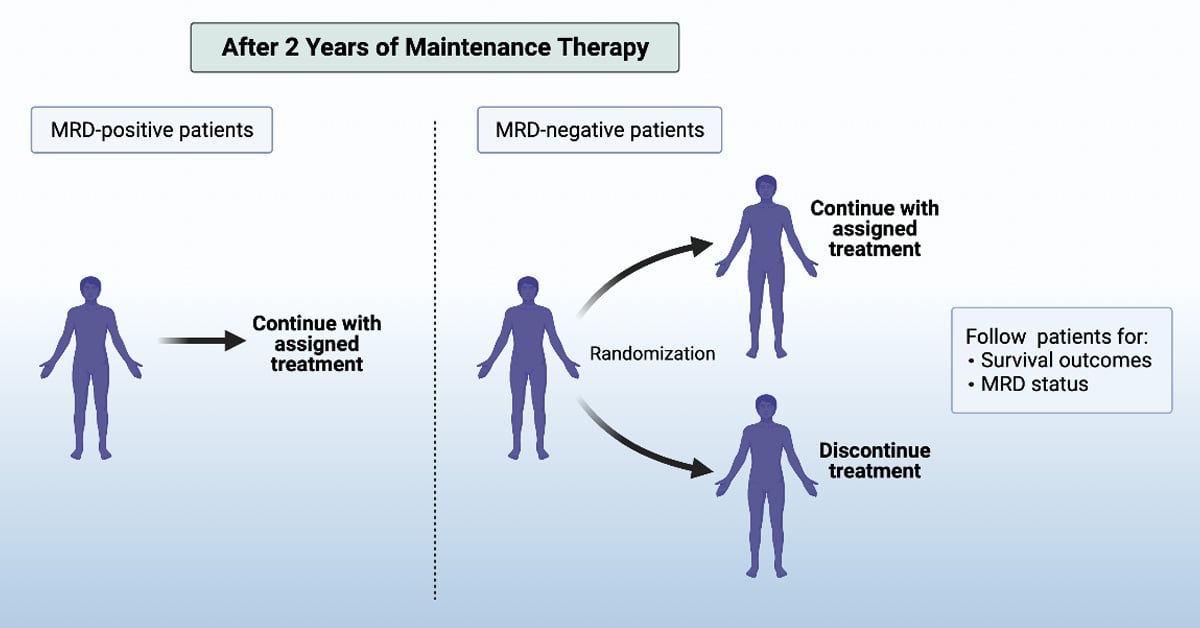
This trial randomizes multiple myeloma patients who have undergone ASCT to either maintenance with lenalidomide (standard of care) versus daratumumab plus lenalidomide. After 2 years of maintenance, MRD (minimal residual disease) is assessed to guide further therapy. MRD-positive patients will continue with the assigned treatment. MRD-negative patients will be further randomized to either continue or discontinue the assigned treatment.
The trial aims to determine if adding daratumumab to lenalidomide as maintenance therapy results in improved overall survival, progression free survival, improved response and MRD negativity in myeloma patients. The first phase of the study will determine if adding daratumumab to current maintenance therapy improves myeloma patient survival outcomes and increases achievement of MRD-negative status. The second phase of the study will assess if maintenance can be safely discontinued when MRD-negative status is achieved at 2 years of maintenance treatment.
Study Overview
This clinical trial offers treatment to patients with multiple myeloma who have undergone ASCT, a type of transplant that uses a patient's own stem cells (cells in the bone marrow that produce new blood cells) to replace those normal cells that are damaged by the high dose chemotherapy treatment that is given to kill cancer cells. Because there is a possibility of cancer returning even after ASCT, patients typically receive maintenance treatment, with the goal of preventing or delaying the return of cancer. In this clinical trial, one group of patients will be randomly assigned to receiving the standard maintenance treatment for multiple myeloma — an immunomodulatory drug called lenalidomide — which works by stimulating cells in the immune system, decreasing the blood supply to tumors, and inhibiting the growth of cancer cells.
Lenalidomide is often given as a single drug on its own. But to find out whether maintenance treatment of multiple myeloma might be more effective when lenalidomide is combined with another drug treatment, a separate group of patients in the clinical trial will receive a combination of lenalidomide and a targeted monoclonal antibody called daratumumab. Daratumumab works by attaching to a protein (the CD38 protein) on the surface of multiple myeloma cells, as well as on other types of cells, such as red blood cells. This allows the drug to directly destroy the cancer cells and/or to help the immune system identify and destroy them. In comparing the two groups of patients, the clinical trial aims to find out whether adding daratumumab to lenalidomide as maintenance treatment will improve the survival of patients with multiple myeloma and help to eliminate minimal residual disease (MRD) — the small number of cancerous cells that can remain even after treatment and that can lead to a return of the cancer.
Another important question that the clinical trial hopes to answer is whether these patients, after 2 years on maintenance treatment, still need to continue with this treatment if they have no MRD. The clinical trial will explore this question in a second part, where patients who have MRD will continue with their assigned treatment (either lenalidomide alone or daratumumab + lenalidomide), while patients without MRD will be further randomized to either continue or discontinue the assigned treatment. The survival outcomes and MRD status of all of these groups will be followed, which will ultimately help determine whether maintenance treatment can be safely discontinued in multiple myeloma patients when MRD is absent after 2 years on maintenance.
"Taking part in clinical trials offers the latest treatment option available to you and helps other patients with a myeloma diagnosis who come after you." - Muthalagu Ramanathan MD
HOW TO ENROLL