in vivo Mechanism of Hsp90
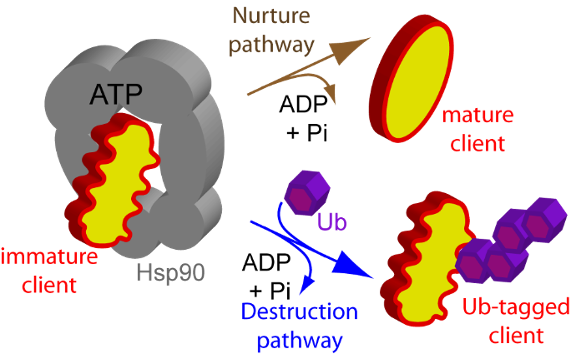
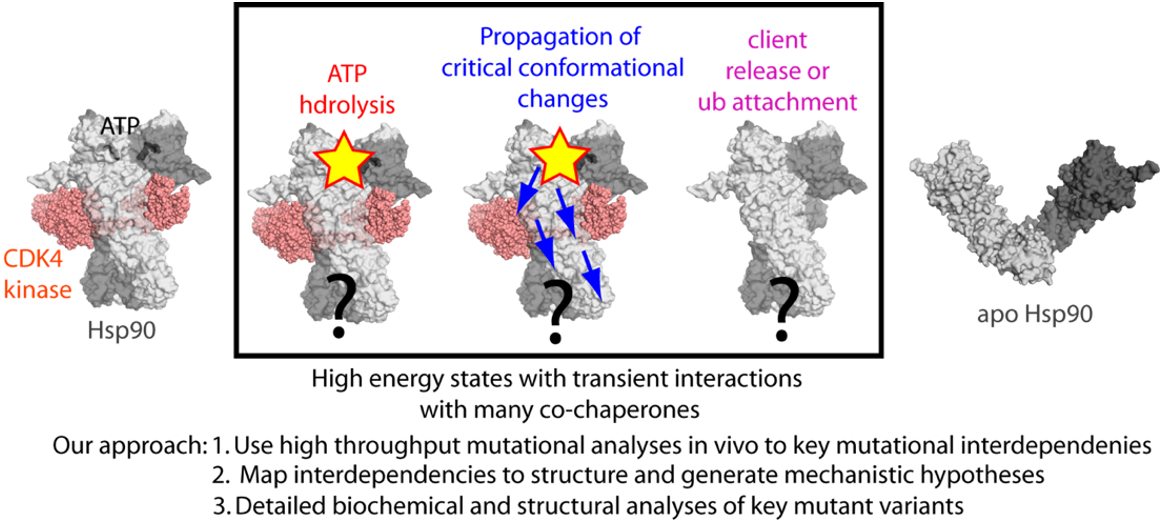
Our research into the in vivo mechanisms of Hsp90 activity aims to answer two questions: (1) how the full-length protein in its native milieu occupies high energy states as it transiently interacts with regulators and clients; and (2) how Hsp90 chooses between the folding or degradation of its clients.
Within the cell, Hsp90 stands out – it is not only one of the most abundant proteins accounting for 1-2% of total cellular protein under normal conditions (and increasing 2 to 10 fold under stress) (Borkovich et al, 1989), but a central hub chaperone that regulates folding, stability, and maturation of more than 300 clients (Schopf et al, 2017). For example, more than half of human kinases bind to Hsp90 (Taipale et al, 2010), and through these kinase clients, Hsp90 mediates most signal transduction pathways in eukaryotes. To achieve the wide range of functions, Hsp90 transiently engages hundreds of other proteins, co-chaperones and client proteins, thus serving as a dynamic protein interaction hub (Kolhe et al, 2023).
Our current work uses methods for in vivo protein analysis to achieve a comprehensive understanding of Hsp90 biology. Our core approach will identify key amino acid interactions that mediate in vivo functions, and we will further examine these variants with detailed biochemical and biophysical approaches. Through these efforts we will obtain deep insights into Hsp90 structure-function relationships in vivo, and capture the “invisible” high energy states that are functionally relevant. We will also dissect how Hsp90 chooses between folding and degradation of the clients. Overall, given that Hsp90 is a central regulatory hub with established links to health and disease, we expect that our results will have significant implications for the field. Importantly, the approaches that we develop in this work are general and can be applied to investigate mechanistic biology of other dynamic hub proteins in vivo.