Research
The goal of the Humphries lab is to elucidate how metabolic pathways can regulate key mediators of cell death and inflammatory responses elicited by our innate immune system.
 Regulation of inflammasomes and cell death
Regulation of inflammasomes and cell death
Inflammasomes are multimeric signaling complexes that sense danger signals in our cells and alert the immune system to clear an infection or respond to stress and tissue damage. Upon activation, inflammasomes trigger a form of cell death known as pyroptosis, where the cell ruptures to release a slew of inflammatory molecules. Our lab is focused on identifying novel mechanisms that control inflammasomes to better understand how we respond to cytosolic danger.
 Metabolic regulation of cell death pathways
Metabolic regulation of cell death pathways
A key goal of our lab is to understand how the oncometabolite fumarate can regulate diverse forms of cell death. Our work is focused on how subversion of cell death pathways by fumarate can regulate tumorigenesis and neuroinflammation.
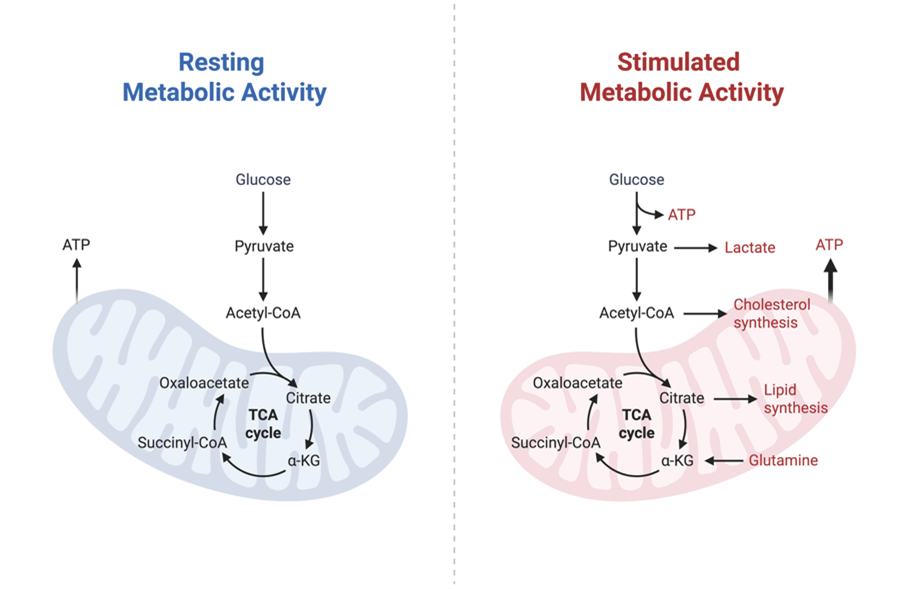 Metabolic Control of viral pathogenesis
Metabolic Control of viral pathogenesis
Like all inflammatory pathways, viral infections trigger metabolic changes in immune cells that can aid and abet viral replication. Respiratory viruses continue to be a global health problem with new and more transmissible variants continuing to emerge. By studying how emerging respiratory viruses shape cell metabolism, we aim to identify new strategies for impairing viral replication and viral entry with the goal of developing new treatments for severe viral infections. Our lab is fully equipped and trained to perform in vitro and in vivo experiments in the BSL3 containment facility at UMass Chan Medical School.
 Therapeutic targeting of innate immunity
Therapeutic targeting of innate immunity
Our lab has a strong focus on translating our findings into novel therapeutics to treat immune-mediated diseases. In line with our other goals, we aim to leverage our findings into new drug modalities for the treatment of inflammatory diseases, cancer, and neurodegeneration. VHH “nanobodies” are a llama-derived class of non-immunogenic, potent, tissue-penetrant antibody with higher specificity when compared to traditional monoclonals. Our lab has identified nanobodies that target key innate immune proteins to regulate immune responses and cell death.