What are toxic metabolites?
Metabolites are the small biomolecules that that serve various functions in a cell. They include building blocks such as amino acids (e.g. proline) or nucleic acids (e.g. deoxycytidine triphosphate) that form macromolecules such as proteins or DNA; substrates for enzymatic modifications such as acetylation or methylation (e.g. acetyl-CoA); signaling molecules such as PIP3. They are made, broken down, or converted from one form to another – in other words ‘metabolized’ – in the cell through sequential enzyme catalyzed reactions called metabolic pathways, and the numerous metabolic intermediates formed during each of these processes are also referred to as metabolites.
When one buys a chemical from Sigma or other vendor, you notice the MSDS safety chart. Toxicity testing must be conducted on any commercially available compound, which includes a large portion of these metabolites – and we begin to notice that many such metabolites have unexpected toxicity. Or that chemicals we utilize and know to be toxic – such as formaldehyde – are actually produced during normal cell metabolic activities. By systematically cataloguing the metabolites formed in our bodies that have toxic properties, we have coined the concept of the ‘endotoxome’, which provides a starting point for utilizing these as poisons to kill cancer cells, or examining them as potential culprits in pathologies such as neurodegeneration.
Why are they toxic?
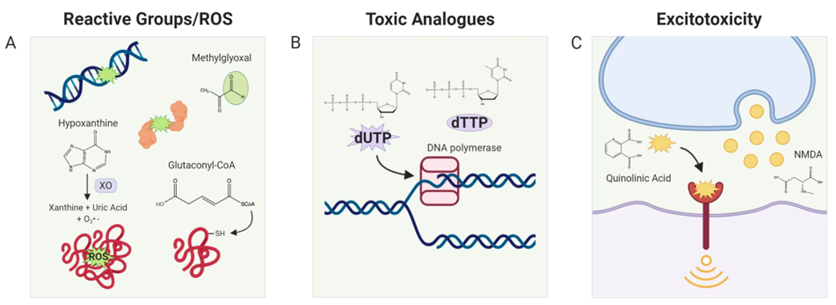
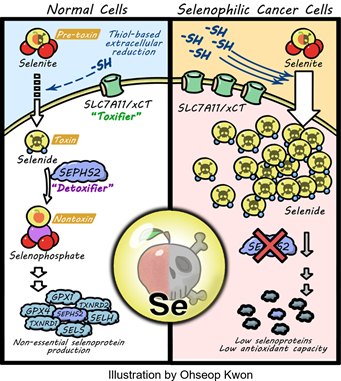
Some toxic metabolites have predictable mechanisms of toxicity. Some are inherently reactive, such as the reactive aldehydes, and will covalently modify proteins and DNA. Some are toxic due to their similarity with other metabolites – for example dUTP, due to its similarity with dTTP, will get mistakenly incorporated by polymerases into DNA. Some are able to erroneously trigger neurotransmitter receptors. But most toxic metabolites have an unknown mechanism of toxicity, which hints at a yet unknown biology of that metabolite, as toxicity indicates interaction of the toxic agent with biological processes. For example, we recently found that the toxic selenium metabolite selenide serves as an electron donor to form the lipid antioxidant ubiquinol in the mitochondria, expanding our knowledge of what roles selenium plays in the body. Along similar lines, our findings suggest that the toxic metabolite 3KDS serves functional roles in ER regulation, and UDPGA regulates aspects of golgi trafficking. In this manner, studying the mechanism of toxicity is an opportunity to uncover the unpredicted biological roles of various metabolic pathways.
Using toxic metabolites to ‘logic gate’ cancer cell death
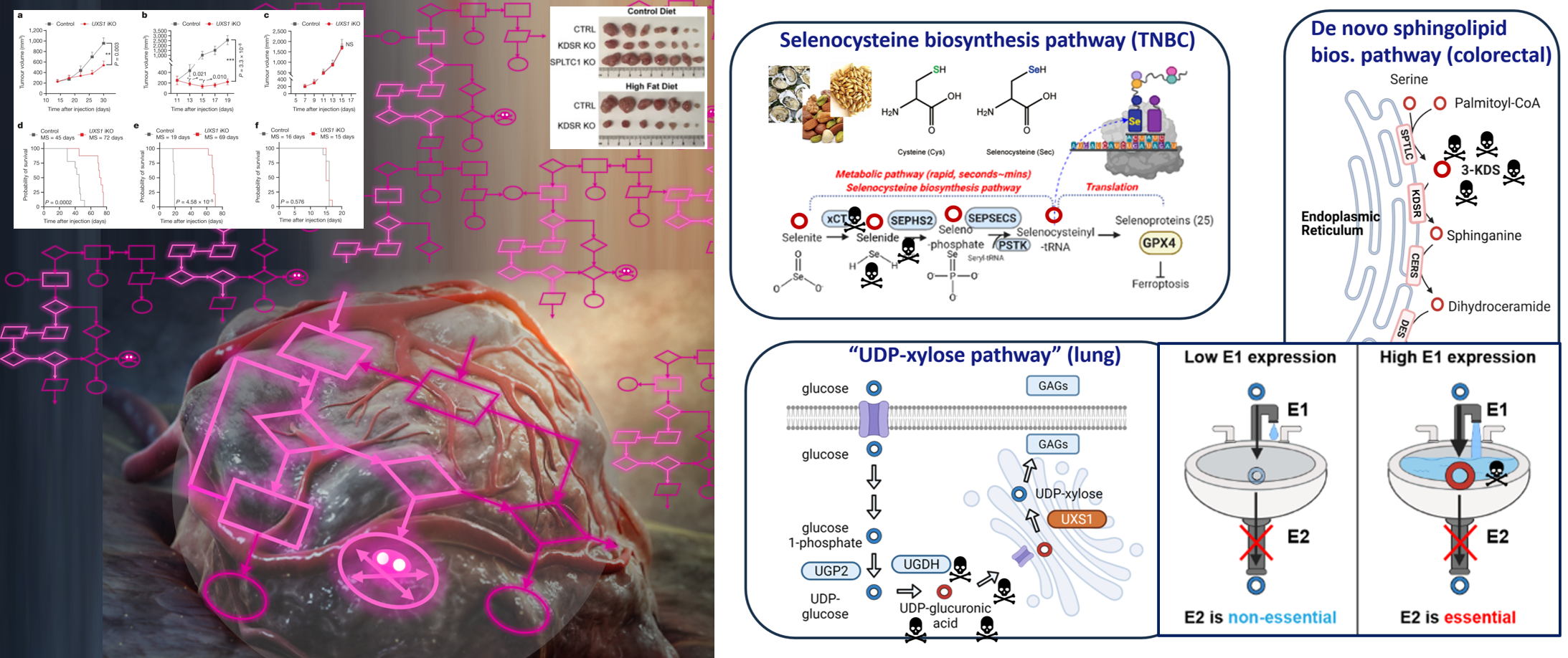
Our studies on toxic metabolites have allowed us to develop a unique, novel approach to selectively kill cells in an algorithm-based manner: by identifying the pathways that are hyperactivated in cancer that also happen to produce toxic metabolites, and targeting the detoxifying enzyme, we uncover novel cancer targets for which we are developing drug inhibitors. Furthermore, we can adjust the toxicity through dietary modulation of the upstream metabolites that feed into toxic metabolite production, and even gain further cancer selectivity when these nutrients are selectively uptaken by cancer cells. We have uncovered novel targets, and strategies to further ‘game the system’ to our advantage via dietary modulation, for breast, lung, and colorectal cancer.
|
Selenocysteine metabolism in cancer |
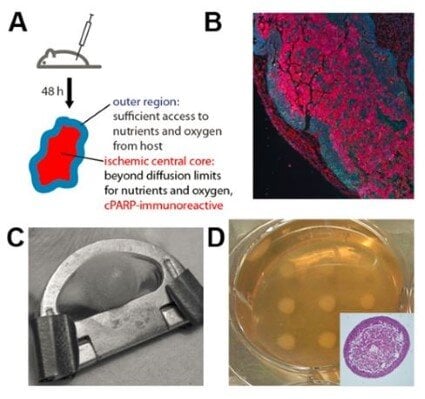
Modeling and characterizing the ischemic tumor metabolic environment |
Investigating toxic metabolite pathways and detoxifying enzymes in cancer |