Innate immune detection of HIV
Boosting cell-intrinsic innate immune recognition of HIV-1 by dendritic cells
There is a pressing need for a vaccine that prevents HIV-1 infection. By conventional criteria, ani-HIV-1 immunity is detected in most people who are HIV-1-infected. Yet, they can be secondarily infected with HIV-1, and, unless treated with anti-retrovirals, they progress to AIDS. Without modifications in vaccine design based on deeper understanding of the immune response to HIV-1, conventional approaches that have worked for other viruses, including attenuated live vaccines, are unlikely to work. Effective antiviral immunity requires that, in addition to presenting viral peptides, and elaborating accessory molecules that stimulate proliferation, dendritic cells (DCs) must provide a third signal (e.g. IL-12) to naïve T cells. There are many ways that a DC can be matured to present antigen, though the outcome of T cell priming may be antigen-specific immunization or antigen-specific tolerance. Generation of the third signal requires that pattern recognition receptors (PRRs) be activated directly within the antigen presenting DC. HIV-1 encounter with DCs results in efficient antigen presentation. But, the DC has multiple blocks to HIV-1 replication, PRRs are not activated, and DC maturation is insufficient to generate the third signal. Hence, the immune response to HIV-1 is not protective, maybe even tolerogenic. The hypotheses to be tested here is that, by increasing PRR signaling with HIV-1-antigen presenting DCs, protective anit-HIV-1 immune responses will be generated.
Aim 1
Aim 1 is to mature monocyte-derived DCs in response to HIV-1 transduction, such that they deliver Signal 3 to naïve T cells. This will be done by modifying the DCs (with knockdown of TREX1 and RNASEH2) and by modifying the virus (incorporating cGAS into the virion and capsid variants that activate TRIM5-dependent signaling). The HIV-1-antigen-specific response of naïve T cells, when primed by these modified DCs transduced modified HIV-1, will be assessed in vitro.
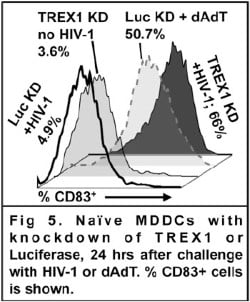
Aim 2
Aim 2 will be to apply the optimized protocols from Aim 1 to fetal liver-derived DCs and test their ability to prime autologous, HIV-1-specific, naïve T cells in vivo, within humanized NSG-BLT mice bearing human transgenes for HLA-A2, IL-7, huBLyS/BAFF, and CSF-1.
Aim 3
Aim 3 will be to determine whether immunization with our modified, HIV-1-transduced DCs offers protection against live HIV-1 challenge, or influences the course of infection, in NSG-BLT mice.
