Structure/function of vertebrate myosin filaments
The skeletal and cardiac muscles of vertebrates are important because of their relevance to human physiology and disease. However, vertebrate filaments are more challenging to study than invertebrates, as they are less stable and more complex (with proteins in addition to myosin, e.g. titin, MyBP-C). Using single particle negative staining EM we initially determined the organization of the myosin heads, titin, and MyBP-C (Zoghbi et al., 2008) in mouse cardiac filaments at 40 Å resolution. Recently, we obtained a high-resolution (6 Å) cryo-EM structure of the human cardiac thick filament, showing the complex arrangement of myosin interacting-heads motifs (IHMs), MyBP-C and titin in stunning detail (Dutta et al., Nature 2023; 623, 853-862). This structure solves numerous questions of thick filament structure that had eluded the muscle field for 60 years, and has become a new paradigm for studies going forward. We are now using cryo-EM to determine the impact of disease mutations and myosin-targeted therapeutic drugs (mavacamten, omecamtiv mecarbil) on IHM structure at ~ 3 Å resolution.(Somavarapu et al., bioRxiv, 2025.2010.2029.685122, doi:10.1101/2025.10.29.685122).
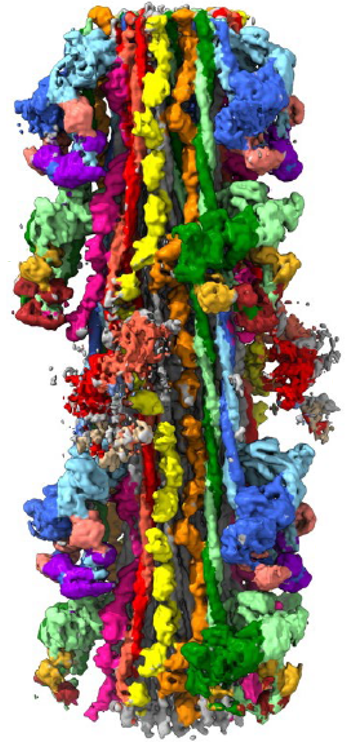
Cryo-EM reconstruction of human cardiac thick filament. Myosin IHMs, blue, green and red, on surface; myosin tails (coiled coil α-helices) in backbone; titin, yellow and orange; MyBP-C, pink.