Structure and function of myosin binding protein C
MyBP-C is a myosin binding protein discovered in the 1970s whose structural organization and function have remained elusive. It is currently a key area of research because of its role in regulating cardiac contraction and in inherited cardiac disease. We collaborated with Dr. Pradeep Luther (Imperial College, London) on electron tomography of cryo-preserved muscle specimens. The tomograms show that MyBP-C is not only bound to the thick filament, but also extends out and connects to the thin filaments (the pink band of density in the figure; Luther et al., PNAS 2011), demonstrating that interactions of MyBP-C with actin observed in vitro are relevant to intact muscle. Bridging between myosin and actin provides a physical basis for MyBP-C’s ability to modulate contraction. We have now observed MyBP-C’s individual C-terminal domains binding to the thick filament surface at 6 Å resolution by cryo-EM (Dutta et al., Nature 2023). The reconstruction reveals unexpected interactions between these C-terminal domains and myosin heads, and thus a novel role of MyBP-C’s C-terminus in regulating contraction.
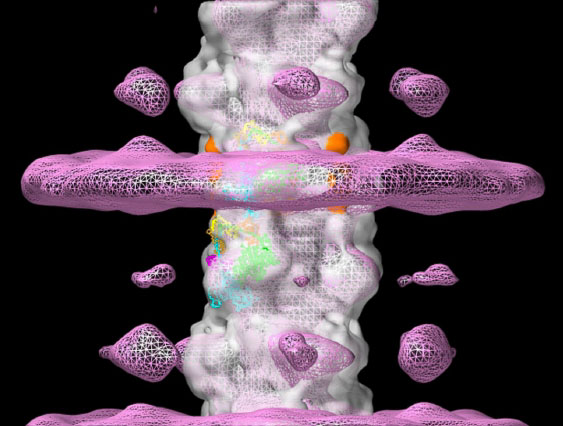
Thick filament tomogram showing MyBP-C extending from thick filament backbone (Luther et al., 2011). Orange shows C-terminal domains of MyBP-C (Zoghbi et al., 2008).
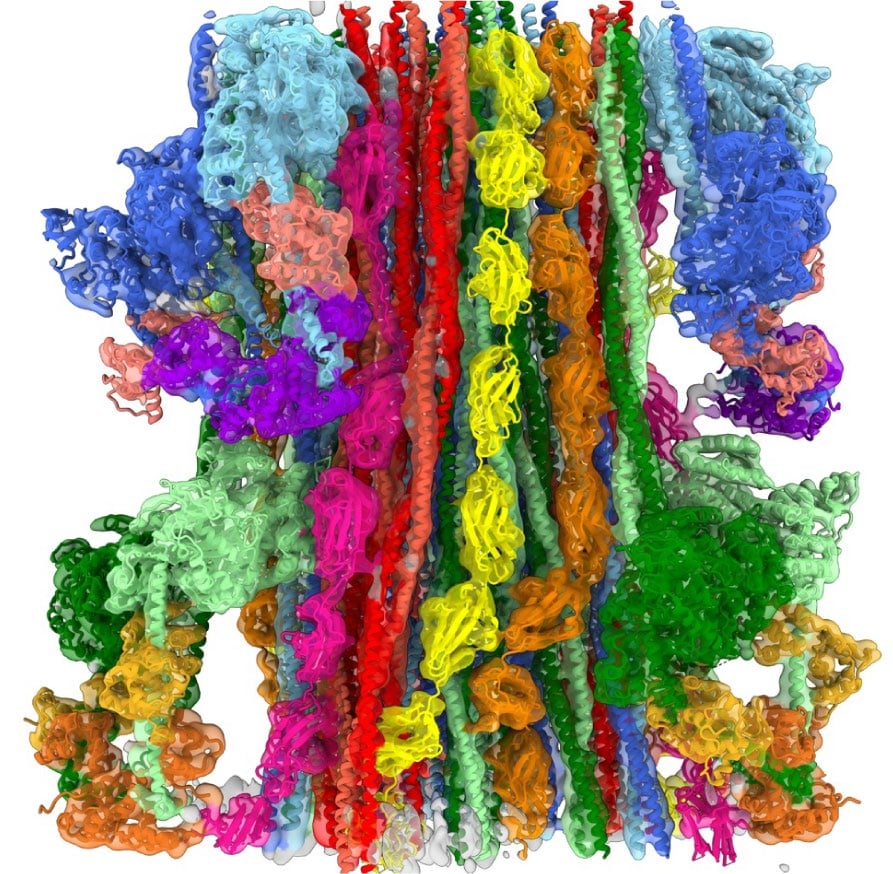
Atomic model of human cardiac myosin filament. C-terminal domains of MyBP-C, interacting with myosin heads, are shown in pink.