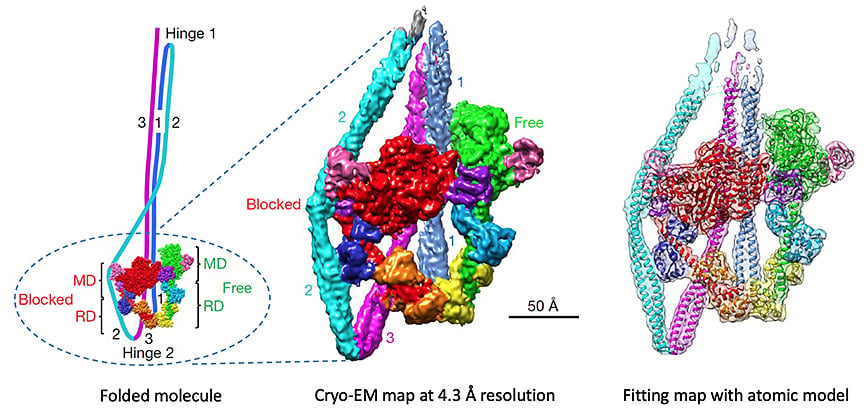
Structure of myosin molecules
The head-head interaction that switches off activity in thick filaments is also found in isolated myosin molecules. This suggests a common mechanism for turning off cell motility and muscle contraction. We used cryo-EM and single particle reconstruction to determine the structural basis of this switching at near-atomic (4.3 Å) resolution (Yang et al., Nature 588: 521–525, 2020). In the off-state, the myosin tail folds into three segments that wrap around the heads. Interaction of the tail with the heads interferes with ATP hydrolysis and actin binding. It also blocks a phosphorylation site required to switch on activity. This completely shuts down activity in the molecule, explaining the extraordinary inhibition of the off-state measured biochemically.