Department News
-

-

Neurobiology Department Food Drive
-

Congratulations Hannah Rogers on receiving a new NIH F31 Fellowship
-

David Dosa and Andrew Tapper named to newly established endowed chairs
-

UMass Chan researchers receive early career awards to conduct studies on biology of aging
-

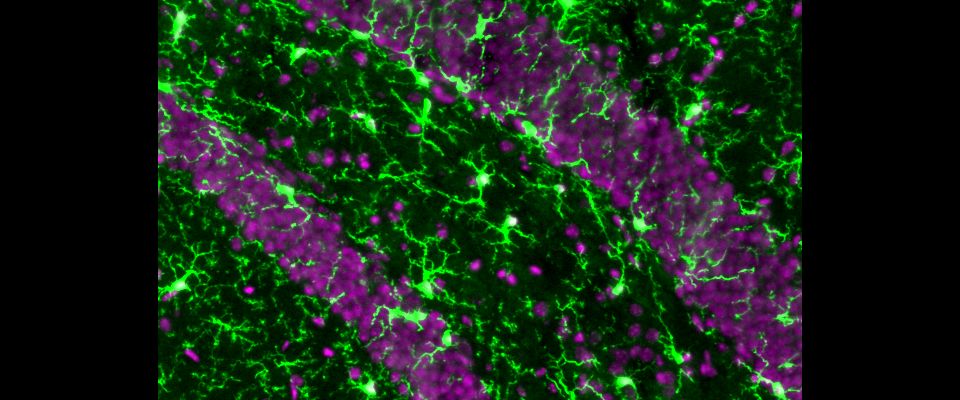
Dorothy P. Schafer receives funding from Cure Alzheimer's Fund
-

Nicholas Bolden MD/PhD Dissertation Defense
-

Logan lab receives three R01 grants for studies targeting opioid use disorder, relapse vulnerability
-

Timmy Le PhD Dissertation Defense
-

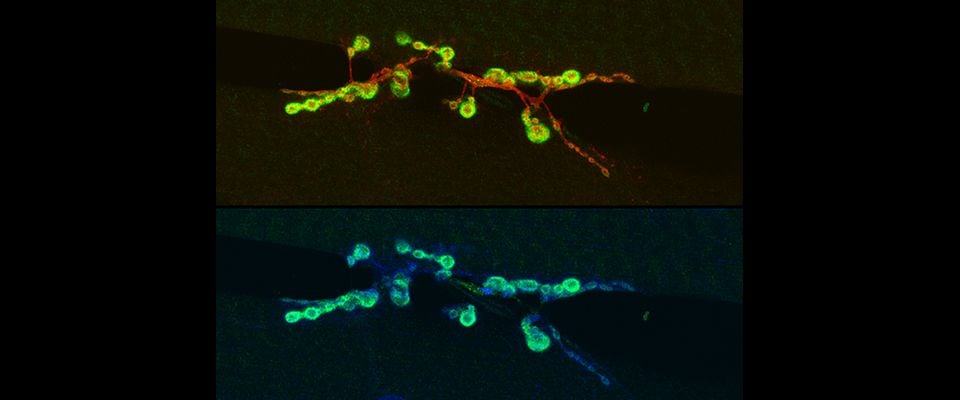
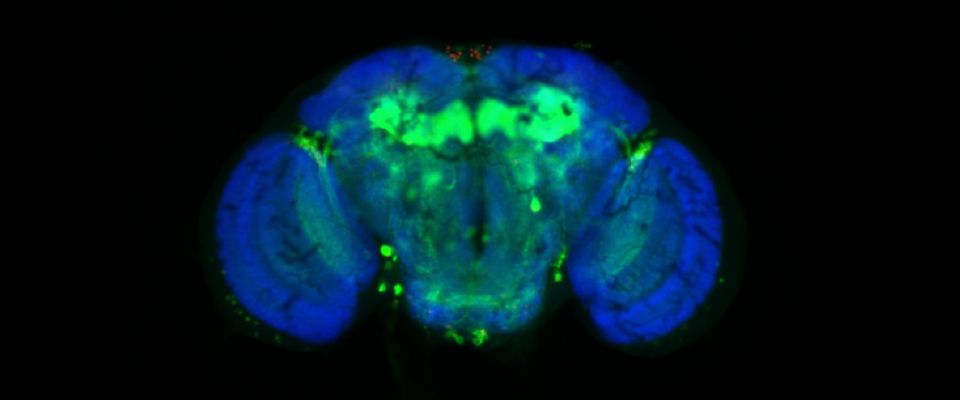
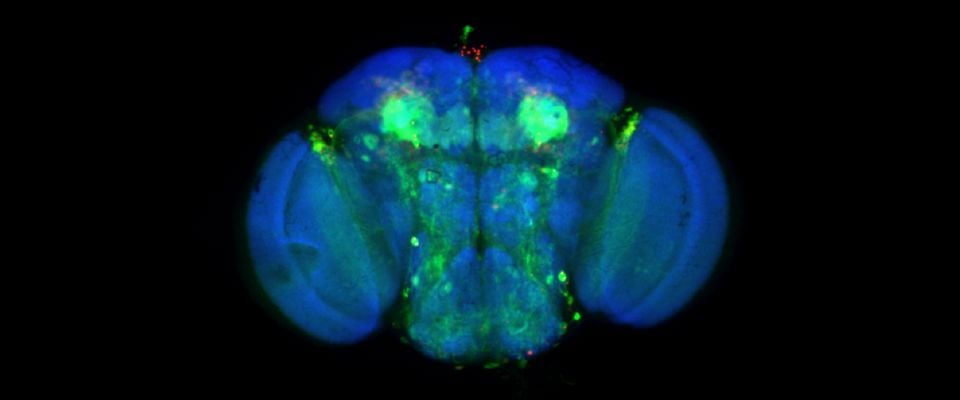
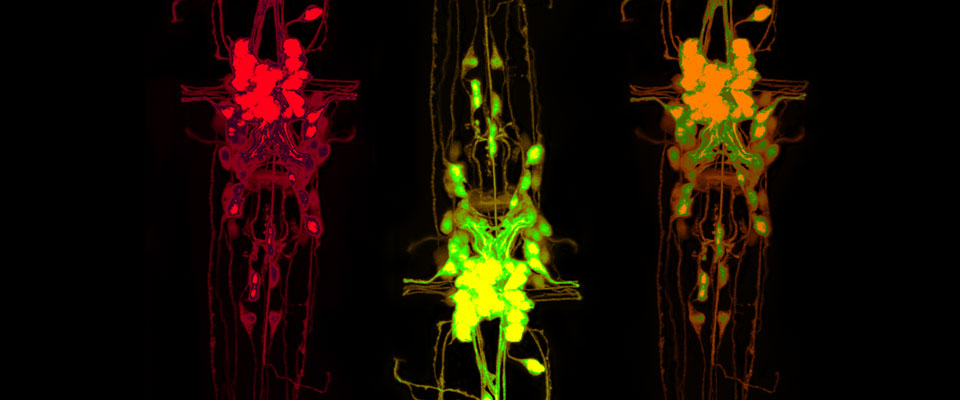
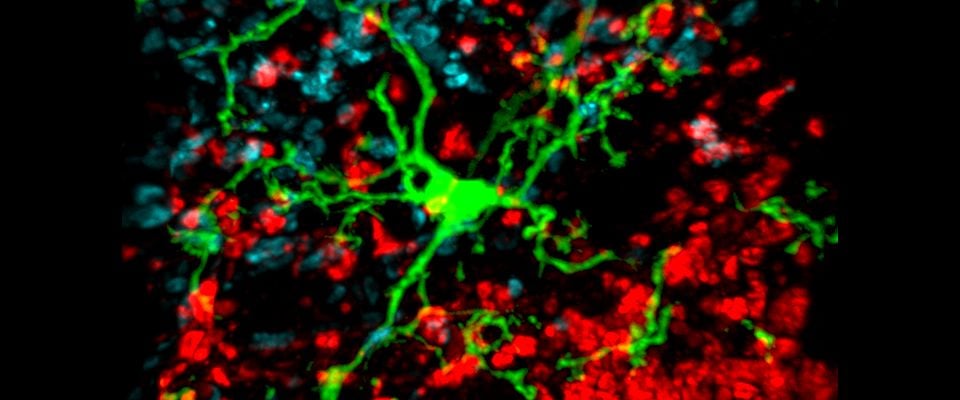
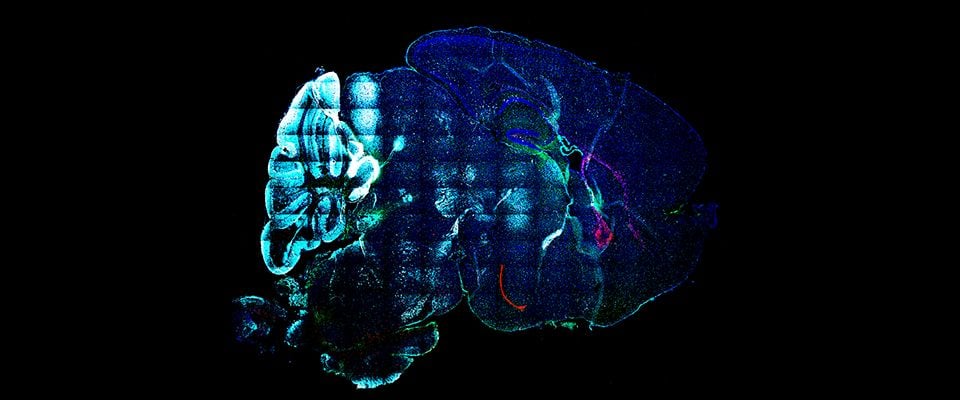
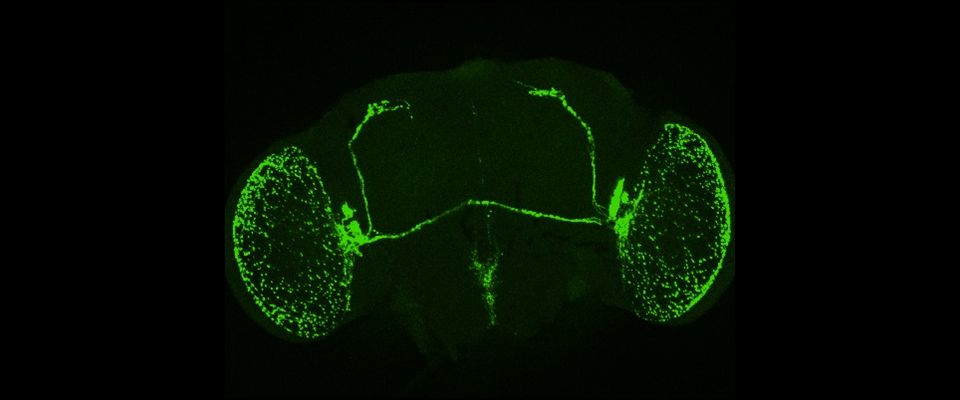
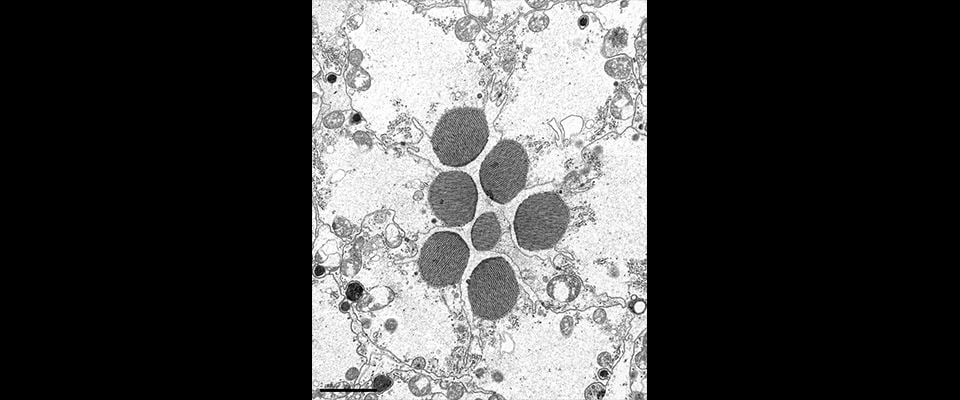
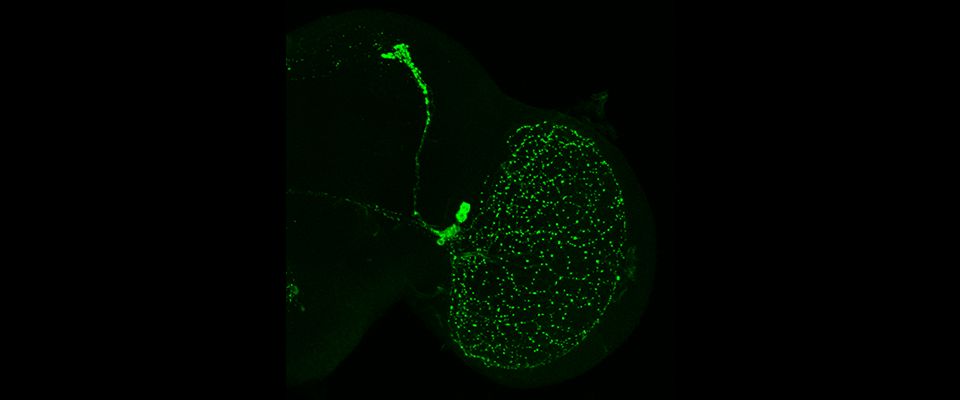
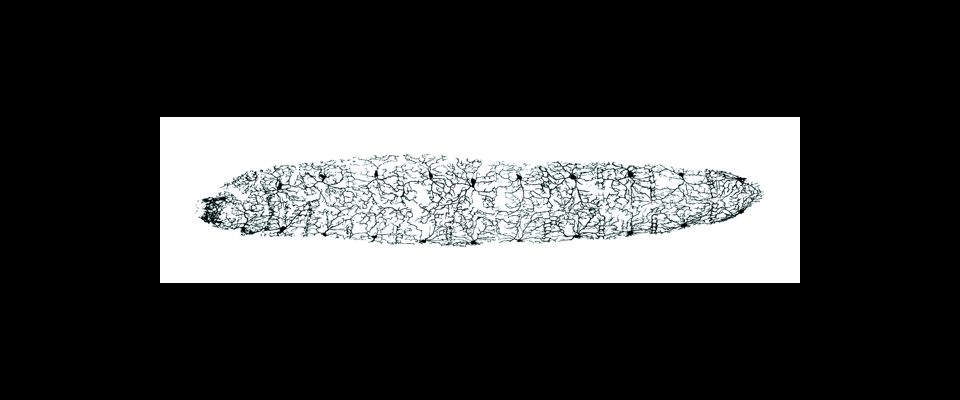
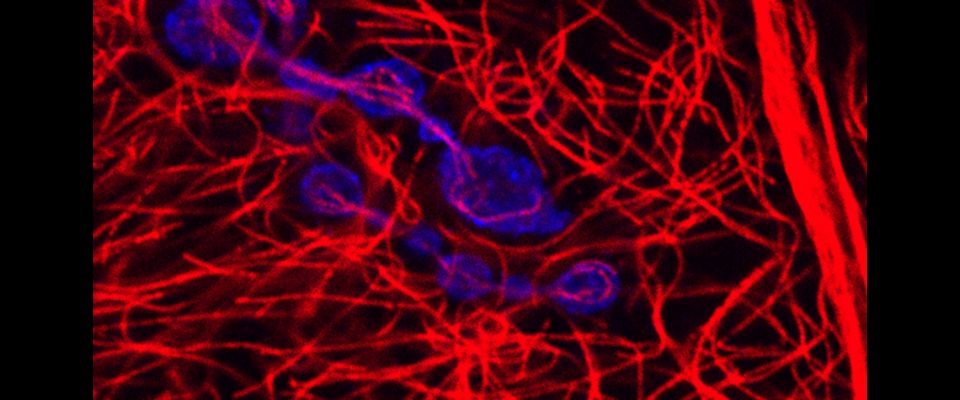
UMass Chan scientists show how non-neuronal brain cells communicate to coordinate rewiring of brain
-

Emily Norman receives the Zelda Haidak award
-

Max Zinter PhD Dissertation Defense