Research Interests
Neural circuit development & function
Our experience of the world around us, and our ability to react to it, are encoded by the connections between the cells of our nervous system (neurons). These connections, known as synapses, organize neurons into dedicated neural circuits that are required for information processing and transfer in the nervous system. Defects in the development and organization of neural circuits are associated with a variety of neurodevelopmental and neuropsychiatric disorders. We are interested in understanding how the synaptic connectivity of neural circuits is established and are working to identify molecular pathways that regulate this process.
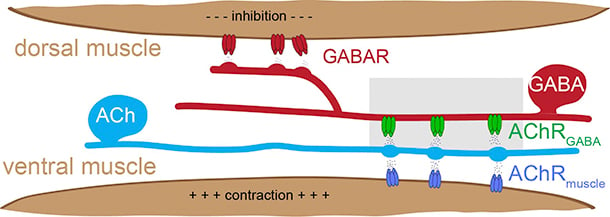
Our studies addressing these questions utilize the experimentally tractable motor circuit of the nematode Caenorhabditis elegans (C. elegans) (Fig.1). In this circuit, a balance of excitatory and inhibitory signaling onto muscles drives coordinated muscle contraction and sinusoidal movement. We are working to define how synaptic connections in this circuit are formed, remodeled during development, and maintained during adulthood. We identified ionotropic nicotinic acetylcholine receptors (iAChR) bearing strong similarity to mammalian brain iAChRs (Fig. 2) that are clustered at synaptic contacts from excitatory neurons onto inhibitory neurons in this circuit. We have developed fluorescent-tagged versions of these receptors and are using these tools to label these synapses in the intact organism. We are using the powerful genetic approaches available in the C. elegans system in combination with in vivo labeling of synaptic connections to identify and investigate molecular mechanisms that shape circuit connectivity.
Molecular mechanisms of developmental circuit remodeling
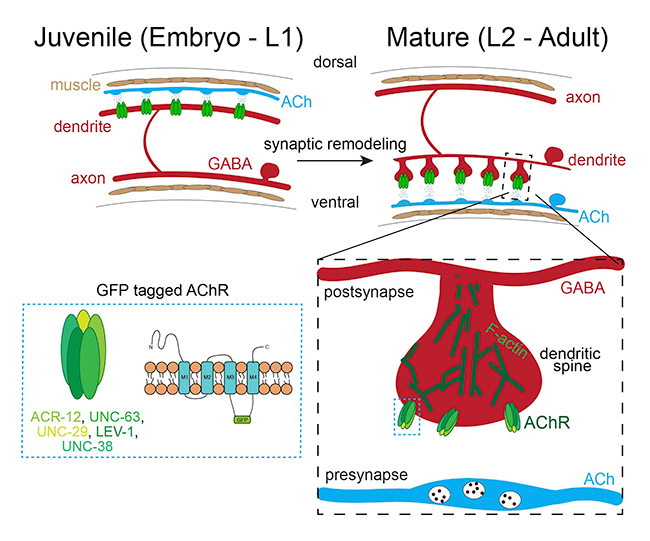
During development and maturation of the nervous system, some synaptic connections are stabilized while others are eliminated. This process, known as synaptic or circuit remodeling, is conserved through phylogeny and is critical for establishing the proper organization and optimal performance of adult circuits. However, we have only a limited understanding of the molecular mechanisms that are responsible. Similar to mammals, adult C. elegans neural connectivity is shaped by developmental processes that modify both the number of synaptic connections and the identity of synaptic partners. Our work seeks to identify and investigate novel mechanisms responsible for synaptic remodeling in the C. elegans motor circuit. Adult connectivity is achieved through a remarkable reorganization of circuit connectivity, most strikingly the remodeling of GABAergic dorsal-D (DD) neuron synaptic connections (Fig. 2). Using the labeling strategy described above, we can monitor the progression of circuit remodeling in vivo (Fig. 3). We are using genetic approaches to identify and investigate molecular mechanisms required for circuit remodeling, in particular for the elimination of juvenile synapses.

Figure 3. GFP-tagged AChRs (green) label synaptic inputs onto GABAergic DD neurons. These inputs are eliminated from dorsal processes of GABAergic DD neurons during remodeling of the motor circuit and relocated to ventral processes. We are working to understand the molecular mechanisms that control synaptic remodeling in vivo.
Synapse formation and maintenance
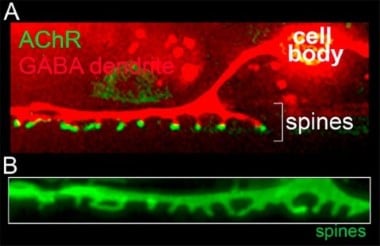
In the adult mammalian brain, a majority of excitatory synaptic connections occur at specialized structures called dendritic spines that serve to compartmentalize biochemical and signaling functions in neurons. Our laboratory identified spiny protrusions from C. elegans GABAergic motor neurons that closely resemble mammalian dendritic spines in their morphology and characteristics (Fig. 4). Using fluorescent labeling of synapses, genetic screens, and live imaging approaches, we have developed a system to monitor synaptogenesis in vivo, with the goal of understanding molecular pathways that regulate spine outgrowth and stabilization. Given the high degree of evolutionary conservation between nematodes and mammals, we expect our work to reveal shared molecular mechanisms.
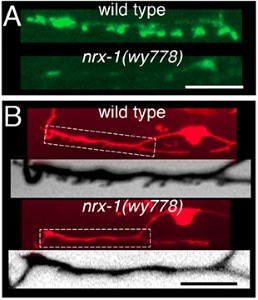
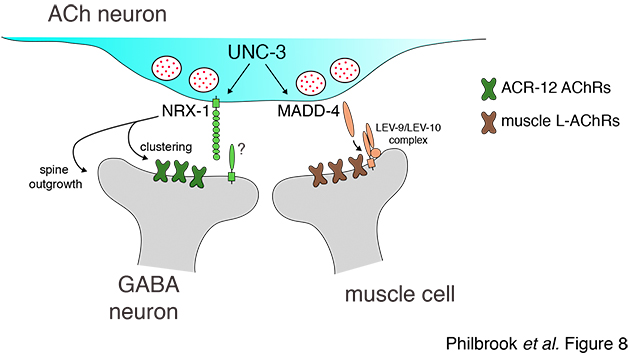
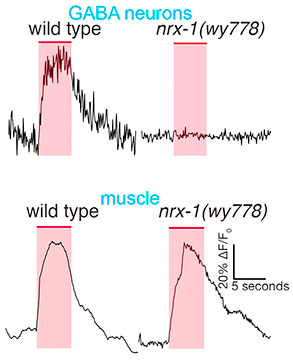
Our initial studies showed that the conserved synaptic adhesion molecule neurexin (C. elegans NRX-1) is required for spine formation or stabilization (Fig. 5). Intriguingly, NRX-1 expression is required in presynaptic motor neurons for postsynaptic spine outgrowth, suggesting a trans-synaptic signaling role (Fig. 6). Though NRX-1 is required for functional synaptic connections between cholinergic and GABAergic motor neurons, NRX-1 is not required for functional connections with muscle cells, the other synaptic partner of cholinergic motor neurons (Fig. 7). Thus, NRX-1 promotes connectivity in a partner-specific manner, offering new insight into how connections from a single neuron onto multiple postsynaptic targets cells are encoded.
Neuromodulation & context-dependent behavior
Information transfer through neural circuits is largely mediated through physical connections between neurons (synapses), but the strength of these connections is often modified by the actions of neuromodulators. Neuromodulators can therefore modify neural circuit activity to elicit alternate outcomes, for example alternate behavior. Sleep, eating, anxiety, mood are among the many behavioral characteristics that are strongly affected by the actions of neuromodulatory systems in our brains. We are seeking to understand how neural circuit activity and behavior is shaped through the actions of neuropeptide modulators. We have been pursuing this question in the context of area-restricted food searching, an ethologically conserved C. elegans behavior program that is triggered by reduced food availability. We identified an important role for the NLP-12 neuropeptide system, a close relative of the cholecystokinin (CCK) system in mammals, in shaping food search behavior (Fig. 8). Our findings show that behavioral responses to reduced food availability are shaped through context-dependent NLP-12/CCK modulation of motor circuit responsiveness to sensory information about food (Fig. 8), a striking example of how the approaches we are pursuing enable us to gain novel insights into signaling pathways directly relevant to mammalian neurobiology. In ongoing work, we are investigating the mechanisms by which NLP-12 signaling regulates food search behavior. In related studies, we are investigating context-dependent neuropeptide modulation in other behavioral paradigms, such as egg-laying.

Figure 8. Context-dependent modulation by NLP-12 neuropeptides promotes food-seeking behavior.
Ion channel-mediated neurodegeneration and neuroprotective mechanisms
The production of reactive oxygen species (ROS) is a major factor influencing cell survival or degeneration in the nervous system. ROS are generated as a by-product of ATP synthesis and can react with DNA, proteins, and lipids, potentially leading to oxidative stress and damage. The human brain accounts for only 2% of the total body mass, yet it utilizes approximately 20% of the body’s total metabolic energy. Neurotransmission, vesicular trafficking and maintenance of ionic gradients require neurons to generate large amounts of ATP and, as a by-product, ROS. Oxidative damage from excess or uncontrolled ROS levels is linked with neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, and ALS, and may contribute to cellular aging. We have only limited understanding of cellular mechanisms for protection from oxidative damage. We have found that that hyperactivation of iAChRs leads to neuron degeneration. We are now working to uncover the molecular pathways that underlie ion channel mediated toxicity, the involvement of ROS, and mechanisms for protection from oxidative damage.