Signaling
While historically signaling pathways and metabolic pathways were often studied separately, it is now clear that signaling and metabolic control are intimately linked. For example, circulating growth factors through cell surface receptors activate intracellular signaling pathways, which promote the uptake and utilization of nutrients. Inside the cell, some bioactive metabolites also function as second messengers that regulate signaling pathways and gene expression. An example is acetyl-CoA, which is both a lipid precursor and the substrate for histone acetylation. We hypothesize that miscommunication between signaling and metabolic pathways triggered by poor nutrition or mutations promotes type 2 diabetes (T2D) and cancer. Therefore, one of our goals is to mechanistically define key points of intersection between nutrient sensitive signaling pathways and the metabolic circuitry of the cell.
Model of how signaling and metabolism can intersect
mTOR signaling
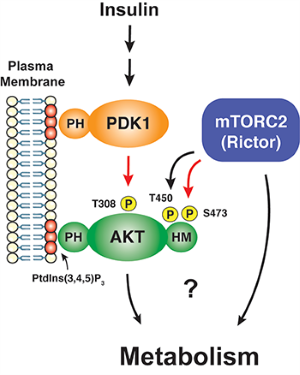
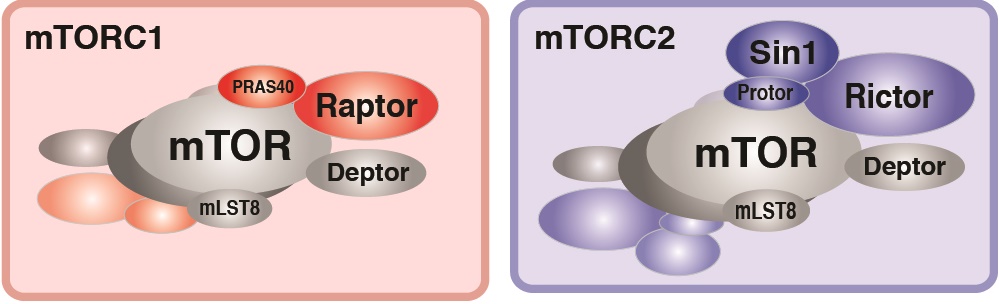
In particular, we have a long-standing interest in the mTOR-signaling pathway. Within cells, the mTOR protein kinase is a critical link between nutrient availability and metabolism. Altered mTOR signaling is associated with cancer, type 2 diabetes, neurological disorders, and many other diseases. Consequently, drugs that target the mTOR pathway are highly sought-after therapeutics. One of the most interesting aspects of mTOR is that its functions are split between two distinct multi-subunit protein complexes, called mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). The complexes have both shared subunits (e.g. mTOR, mLST8) and unique subunits (e.g. Raptor in mTORC1 and Rictor and Sin1 in mTORC2). Each mTOR complex senses different upstream signals, localizes to different places in cells, and has different downstream targets. How these diverse mTOR functions are coordinated in cellular time and space to control metabolism remains poorly understood, especially in vivo.
Click here to view a model of the mTOR signaling pathway
The least understood mTOR complex is mTORC2. Through biochemical and genetic studies by our lab and others we have learned that mTORC2 is essential for the growth of many tumors, especially those with activating mutations in the PI3-kinase pathway. Moreover, in major metabolic tissues, such as adipose tissue (fat), mTORC2 coordinates carbohydrate and lipid metabolism. The latter function is critical for regulating whole body metabolic homeostasis indicated by the fact that mice lacking mTORC2 in adipose tissue develop severe hepatic insulin resistance. mTORC2 is also required for normal carbohydrate and lipid metabolism in the liver and has important roles in the brain and immune system that are just beginning to be explored.
The best understood mTORC2 substrate is the AKT kinase, which has broad roles in cell growth, proliferation, survival and metabolism. However, unlike its sibling, mTORC1, we know very little about what regulates mTORC2 activity, where it functions in the cell, and what its downstream functions are beyond AKT. Even its role in AKT signaling remains murky. Resolving the latter is particularly important because mTORC2-dependent AKT phosphorylation predicts poor prognosis in cancer and is the major cellular biomarker of insulin sensitivity in T2D models. We are using a combination of approaches ranging from structural biology, biochemical assays, and cell biology to omics and animal modeling to explore mTORC2’s mechanisms of regulation and function. We hope this will allow us to better understand its role in human disease.