Research
Bitter taste receptors (TAS2Rs) in extra-oral tissues
Bitter taste, one of five basic taste qualities, guides organisms to avoid harmful toxins and noxious substances and is critical to animal and human survival. It has long been thought that bitter taste receptors in the specialized epithelial cells in the tongue's taste buds detect bitter tastants and initiate the sensation of bitterness. We recently discovered that bitter taste receptors are expressed in the airway and uterine smooth muscle, and bitter tastants can relax these muscles more completely than currently used bronchodilators or tocolytics (Zhang et al., 2012; Zheng et al., 2017). We further uncovered that G protein gustducin’s βγ subunits play a critical role in mediating airway relaxation induced by bitter tastants (Zhang et al., 2013). Our work raises the possibility that the evolution of TAS2Rs may also be driven by the selection pressure on bitter tastant-induced physiological functions in the extraoral tissues/organs. We are continually working to understand the molecular mechanisms by which bitter tastants relax uterine and airway smooth muscle. All living organisms (bacteria, plants, and animals) produce bitter tastants, and many current drugs taste bitter. We are interested in finding endogenous ligands for TAS2Rs and bitterness-based medicines for human health and disease.
Local Ca2+ signaling
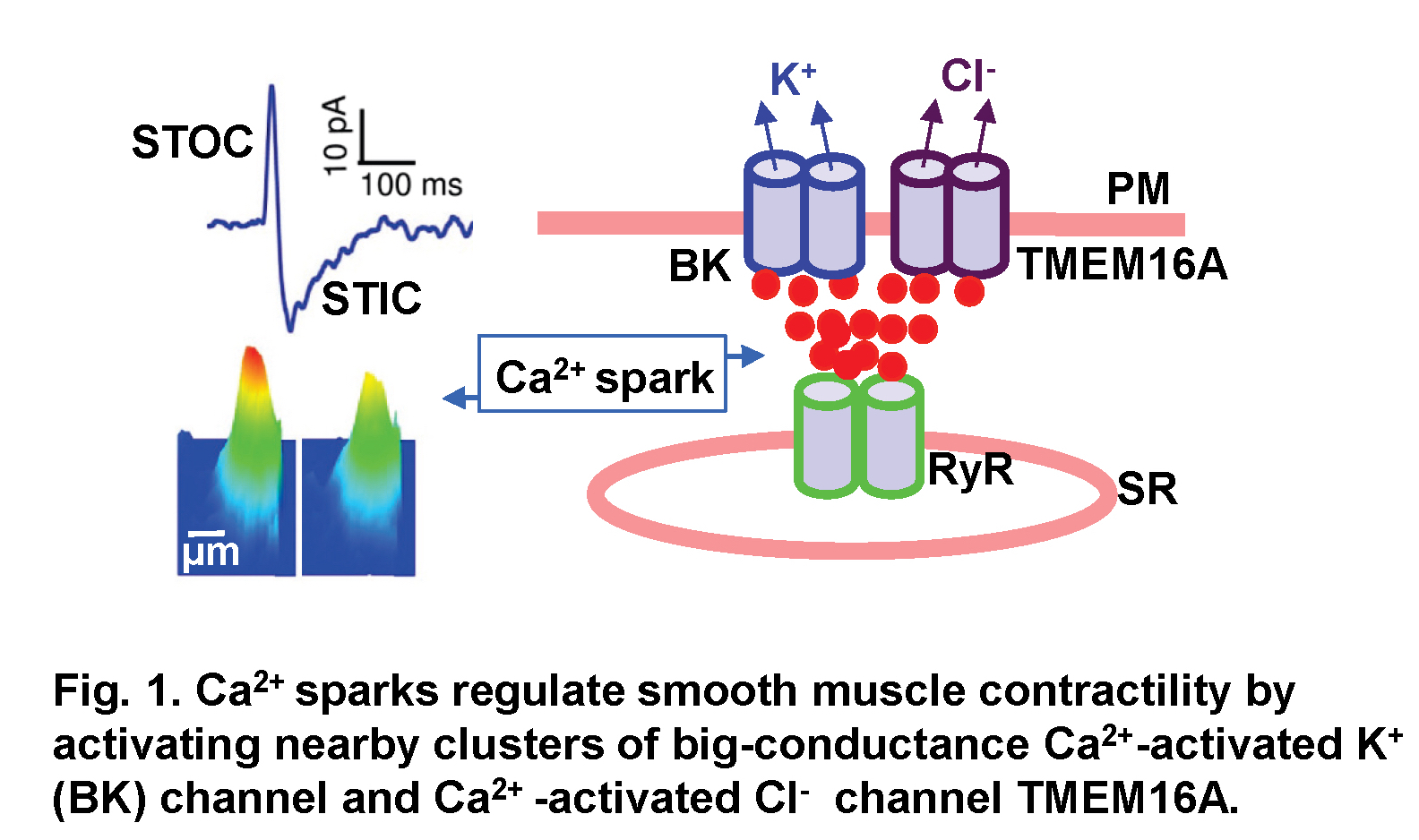
Ca2+ is a universal signaling ion for both life and death. Its concentration within the cell is precisely controlled and regulated in time and space. We have been studying localized, short-lived Ca2+ transients (Ca2+ sparks) that result from the opening of a few clustered ryanodine receptors (RyRs) in the membrane of the sarcoplasmic reticulum. These local Ca2+ signals are the elementary events of precipitating global changes of Ca2+ in striated muscles. In smooth muscle from airways, corpora cavernosa, and some blood vessels, Ca2+ sparks act in their own right to turn on a cluster of big-conductance Ca2+-activated K+ (BK) channels and Ca2+-activated Cl- (Cl(Ca)) channels in the vicinity of release sites (Fig. 1). Activation of these two types of channels produces spontaneous transient outward currents (STOCs) (Video 1) and spontaneous transient inward currents (STICs), respectively, which regulate the activities of voltage-dependent Ca2+-permeable channels. We recently discovered that Ca2+ sparks function as stabilizers of membrane potential and control the contractile state of airway smooth muscle, and Cl(Ca) channel TMEM16A is up-regulated in a mouse model of chronic asthma. We aim to understand the mechanisms by which Ca2+ sparks activate BK and TMEM16A Cl(Ca) channels, investigate the structure and function of TMEM16A, and determine the roles of Ca2+ spark signaling in asthma.
Neurotransmitters transmit the signals from a neuron to its target neurons or non-neuronal cells. It is known that neurotransmitter release is triggered by Ca2+ influx through voltage-dependent Ca2+ channels. However, it remains elusive how Ca2+ releases from internal Ca2+ stores affect neurotransmitter release. Using adrenal chromaffin cells, we discovered that these cells predominantly express RyR2, the cardiac isoform of RyR, and their opening generates localized Ca2+ events, designated as Ca2+ syntillas (De Crescenzo et al. 2004). We also found that Ca2+ syntillas do not trigger catecholamine release (ZhuGe et al. 2006) as current neurotransmitter release dogma would predict. We further demonstrated that Ca2+ syntillas suppress spontaneous catecholamine release (Lefkowitz et al. 2009). Also, action potentials at physiological frequency release catecholamine, in large part due to their inhibition of Ca2+ syntillas (Lefkowitz et al., 2014). So our studies have revealed a new mechanism for the regulation of neurotransmitter release. Since catecholamine mediates many fundamental physiologic responses such as the “fight or flight” stress response, studying this mechanism could produce a new understanding of these essential biological functions.
Molecular mechanisms controlling the tone of smooth muscle sphincters
Smooth muscle sphincters are ring-shaped structures encircling an opening or passage in hollow organs. These structures control the entrance or release of contents in these organs, mediating various biological functions essential for homeostasis. Physiologically, they often remain closed most of the time and open for a short period as needed. Defects in sphincters cause a variety of diseases, including gastroesophageal reflux disease and fecal/urinary incontinence. A central unsolved question in the field of smooth muscle has been how smooth muscle cells generate a spontaneous or basal tone to maintain sphincters in the contracted state. We have used internal anal sphincter (IAS) as a phenotype to understand the molecular mechanisms underlying the basal tone. Using combined techniques from molecular biology, biophysics, and physiology, we have established that the interplay of the RyR-TMEM16A and L-type voltage-dependent Ca2+ channel raises the intracellular Ca2+ concentration, leading to the activation of myosin light chain kinase and the generation and maintenance of IAS basal tone (Zhang et al., 2016). We use a state-of-the-art two-photon microscope to understand synchronized Ca2+ oscillations and asynchronized Ca2+ oscillations in IAS tissues (Lu et al., 2021).
Uterine peristalsis and its roles in reproduction and gynecological disorders
Uterine peristalsis, a unique motility behavior in the non-pregnant uterus of female mammals and humans, plays critical roles in menstruation shedding, sperm and embryo transport, and implantation. Its hyperactivity has been associated with adenomyosis, endometriosis, and leiomyomas, resulting in dysmenorrhea, heavy menstrual bleeding, pelvic pain, infertility, and pregnancy complications. However, the molecular mechanisms on the uterine peristalsis genesis and this contraction's roles on reproduction and gynecological disorders are poorly understood. We use genetic, imaging, and physiological approaches to identify the gene(s) in uterine smooth muscle cells required for uterine peristalsis generation and determine whether these genes contribute to or are responsible for adenomyosis.