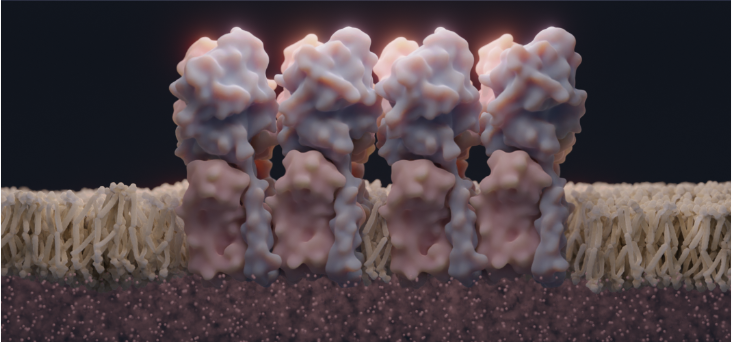
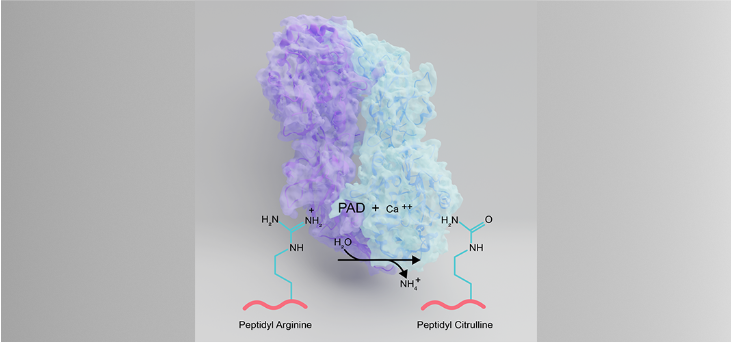
Diseases like rheumatoid arthritis and cancer affect millions every year, and our studies could help alleviate human suffering by identifying new drugs.

Our lab uses kinetic and structural analyses to guide the design of small molecule inhibitors, which we then synthesize and evaluate as inhibitors.
Fuhrmann, J., Clancy, K., Thompson, P.R.
(2015) Rev. 115 , 5413-5461. PMC4463550
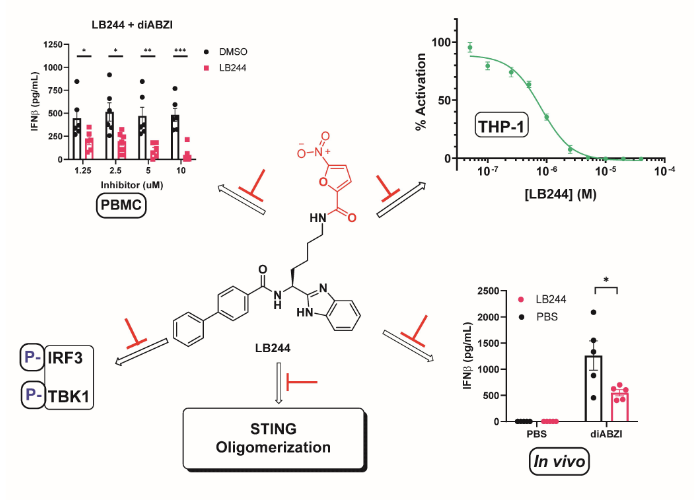
Humphries, F., Shmuel-Galia, L., Jiang, Z., Zhou, J., Barasa, L., Mondal, S., Wilson, R, Sultana, N., Shaffer, S.A., Ng, S., Pesiridis, G.S., Thompson, P.R.* and Fitzgerald, K.A. *
(2023) Targeting STING oligomerization with small-molecule inhibitors. PNAS 120, e2305420120.
Mondal, S., Chen, Y., Lockbaum, G.J., Sen, S., Chaudhuri, S. Reyes, A.C., Lee, J.M., Kaur, A.N., Sultana, N. Cameron, M.D., Shaffer, S.A., Schiffer, C.A., Fitzgerald, K.A., and Thompson, P.R.
(2022) J. Am Chem Soc. 144, 21305-21045. PMID: 36356199. PMC9662648
Mondal, S., Wang, S., Zheng, Y., Sen, S., Chatterjee, A., and Thompson, P. R.
(2021) Nature Comm. 12, 45. PMID: 33398026