 |
|
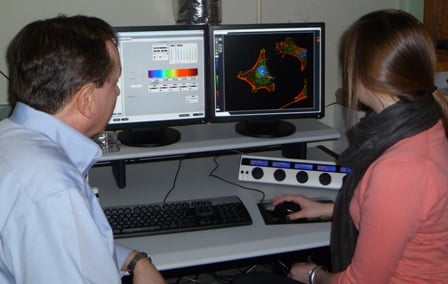
| Jeffrey Nickerson, PhD (left), and Nikki Rossetti examine three-dimensional cellular images displayed on the confocal laser microscope console | |
Last summer, Wellesley College senior Nikki Rossetti made a significant scientific discovery in a UMass Medical School laboratory, a somewhat unusual achievement for an undergraduate. She did this by using a state-of-the-art research tool called a three-dimensional laser confocal microscope. “It was exciting to use microscopy in a new way and actually generate results,” said Rossetti, a neuroscience major who plans to pursue a career in biomedical research. “It was an ‘aha’ moment.”
Rossetti conducted her research as a member of the UMMS Summer Undergraduate Research Program Class of 2011 in the laboratory of Jeffrey Nickerson, PhD, associate professor of cell biology. “We do microscopy to see what things look like and to understand the mechanisms by which they work. We do three-dimensional microscopy because life is three-dimensional,” said Dr. Nickerson, who directs the Cell Biology Three-Dimensional Microscopy Laboratory (3DML) core facility.
While traditional light microscopes can only capture two-dimensional images, one at a time, of a cell, organism or tissue that has been manually cut into very thin sections—a difficult and tedious process—the confocal microscope creates a sharp focal point by channeling light through a small pinhole. The focal point remains stationery while a motorized container attached to the microscope moves samples beneath it. Called optical sectioning, this process produces a series of highly precise, well focused images that show structures in three dimensions and movement in real time.
Using a confocal microscopy technique called fluorescent recovery after photobleaching (FRAP), Rossetti worked with Alexandre Quaresma, PhD, postdoctoral fellow in the Nickerson lab, to measure the binding and transport—essential processes of cell birth, development and death—in certain proteins in cell nuclei that are involved in the export of the genetic building block messenger RNA (mRNA) from the nucleus to the cytoplasm. They then treated the cells with different drug compounds that changed their transport and binding dynamics, to find out which signal transduction pathway—a series of chemical reactions by which molecules inside the cell can be altered by molecules on the outside—might actually regulate the binding of mRNA export. Their results identified two pathways that are targets of cancer chemotherapy.
“Because the particular protein that Nikki was studying is a candidate chemotherapy agent, learning that it has an effect on the fundamental cellular processes of binding and transport may have implications for understanding the development of cancer cells, and how drugs work to kill them,” said Nickerson. Rossetti’s poster presentation mRNA Nuclear Export Protein Binding is Regulated by the Phosphatidylinositol Signaling Pathway in Live Cells was named the best in the class.
Using 3-D, real time imagery to find clues to the progression and treatment of cancer is just one of the confocal microscope’s many research applications. The technology is available to all students and scientists at UMMS, new and experienced alike. Established by Nickerson in 1999 with the purchase of the institution’s first 3D laser confocal microscope, the core now boasts a brand new machine, the Leica SP5 II, bringing the latest refinements in the technology to researchers. The equipment is available for use 24 hours a day, seven days a week, with training provided for graduate students, research associates and faculty who are new users, and ongoing technical support for the many labs in the Department of Cell Biology and other departments that are already taking advantage of the resource.
“We have witnessed an explosion of new microscopy technologies in recent years and are only beginning to exploit them,” Nickerson wrote in the journal RNA Biology about confocal microscopy techniques including FRAP. “Advances in microscopy may change the very way in which we think about biology.”
View the video below to see the confocal microscope in action. In the experiment, “Transiently transfected U2OS cells expressing human protein RUNX2 fused to EGFP,” the time-lapse movie shows the localization of this human protein over mitosis, which is the usual method by which cells divide.
Related links:
Cell Biology Confocal Core—Three Dimensional Microscopy