Post Translational N-Glycosylation
What is Post-translational N-Glycosylation?
The vast majority of N-glycoproteins acquire their initial N-glycans co-translationally during insertion into the endoplasmic reticulum (ER). However, there are a subset of N-glycosylation sites (NXT or NXS) that are skipped by the oligosaccharyltransferase (OST) that is docked to the ER translocon (Sec 61). Our laboratory serendipitously discovered that the KCNE1 (E1) regulatory subunit acquires one N-glycan co-translationally and one N-glycan post-translationally. By examining the KCNE family of type I transmembrane peptides, we identified several molecular determinants that define whether an N-glycosylation site is modified co- or post-translationally. siRNA experiments demonstrated that the STT3B OST isoform was responsible for post-translationally modifying KCNE subunits.
Mutations that affect N-Glycosylation rates cause Long QT Syndrome.
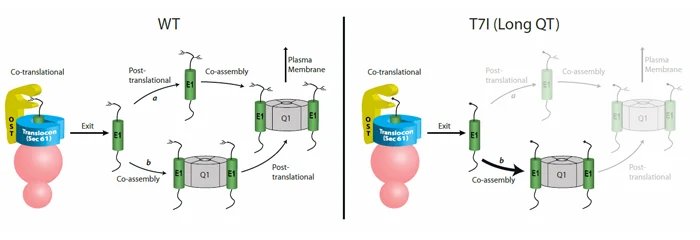
Efficient post-translational N-glycosylation of KCNE1 subunits is essential for their biogenesis and trafficking to the plasma membrane. We determined the molecular mechanism of a KCNE1 mutation (T7I) that causes fatal cardiac arrhythmias (cartoon). WT KCNE1 subunits acquire one N-glycan co-translationally and one N-glycan post-translationally (left panel). KCNQ1/KCNE1 potassium complexes that receive some N-glycosylation are able to leave the ER and traffic to the plasma membrane. In contrast, the T7I mutant does not receive an N-glycan during translation and it exits the ER translocon unglycosylated. Unglycosylated KCNE1 subunits are not post-translationally glycosylated and thus assemble with KCNQ1 channel subunits impeding the complex from reaching the plasma membrane.
New Directions in N-glycosylation:
Are there disease-causing mutations that cause collateral damage to post-translational sites? Why does the STT3A isoform skip certain sites? Can we build a better or a version that skips different sites? What happens to the N-glycosylation sites in potassium conducting subunits? Are the molecular determinants different? Do the same OST isoforms do the same job on these polytopic membrane proteins? What happens in cardiomyocytes?