Identifying Genes and Signaling Pathways to Develop Thymus Cells to Explore the Root Cause of Type 1 Diabetes in the Maehr Lab
Date Posted: Thursday, February 03, 2022
The Maehr laboratory in the UMass Diabetes Center of Excellence is focused on the thymus, the major organ involved in educating the immune system. Within the thymus, thymus cell lymphocytes or T cells mature. Those are the cells that destroy the insulin-producing beta cells in people with Type 1 diabetes (T1D). The Maehr Lab is using human pluripotent stem cells to develop functional thymic epithelial cells that will be used to study the role the organ plays in that autoimmune attack.
Cell differentiation is the process in which cells become specialized. Scientists in the Maehr Lab are replicating that process by developing thymic epithelial cells in a dish.
PhD candidate Margaret Magaletta and bioinformaticians Macrina Lobo and Eric Kernfeld co-authored a newly published study in Nature Communications that examined changes in gene expression and cellular behaviors that occur during the development of several organ domains including the thymus.
The Study
Integration of single-cell transcriptomes and chromatin landscapes reveals regulatory programs driving pharyngeal organ development
Margaret E. Magaletta, Macrina Lobo, Eric M. Kernfeld, Hananeh Aliee, Jack D. Huey, Teagan J. Parsons, Fabian J. Theis & René Maehr
“This data is a culmination of collaborative efforts that began in 2018,” said René Maehr, PhD, Associate Professor at UMass Chan Medical School. “It successfully advanced our investigation into thymus development.”
They used mice for their study subjects since there’s significant overlap in the genes expressed during both mouse and human development. The emergence of different cell types (i.e. thymus, parathyroid, thyroid, ultimobranchial body, esophagus, etc.) was occurring between 11-12 days in the mice, which roughly equates to a 7 week old human embryo.
“We took an all-encompassing approach to understand the genes that are expressed during early thymus development,” said Magaletta. “Observing how those cells change over time will help us to replicate the process with our stem cell differentiation approaches.”
They used two techniques in tandem to analyze the cells. Single cell RNA sequencing showed which genes were activated during the formation of thymus and other cell types. Single cell ATAC (Assay for Transposase Accessible Chromatin) identified regions in the genome that may be involved in regulating expression of the genes.
“We’ve produced an in-depth profile of the epithelial cell types in the embryonic pharynx using these two approaches to understand how the different cell types are forming within one tissue,” added Magaletta.
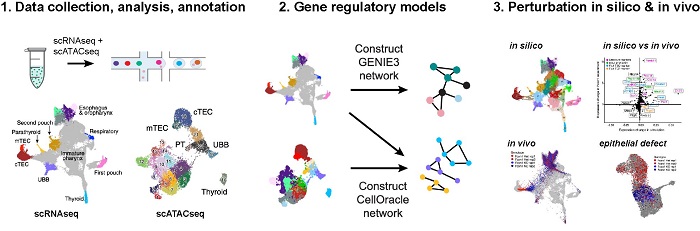
The scientists profiled multiple cell types to provide context and to compare what makes the thymus cells unique.
“When we’re emulating development of cells in the dish, if they veer towards thyroid, parathyroid or other cells, having that comparative information can help us correct it to achieve our goal of creating thymic cells,” said Lobo.
Their data analysis indicated that the gene Foxn1, which had already been linked to thymus development, was a top factor of genetic regulation. They predicted that knocking out Foxn1 would create a developmental delay in the thymus, and analysis of the Foxn1 mutant indeed showed an immature gene expression profile compared to the control.
“Foxn1 is required for proper development of the thymus, so mutations in this gene can severely impact the ability of the thymus to produce self-tolerant T cells,” said Magaletta. “We wanted to fully understand the changes that occur in thymus development when you lose that gene.”
Foxn1 deficiency has also been linked to alopecia, nail dystrophy and severe combined Immunodeficiency (SCID).
Next Steps
The Maehr Lab has been using this data as a blueprint for future tissue engineering in the lab.
“In recent years we’ve developed a 3D model of the thymus and the information presented in this latest paper brings us another step closer to creating a functional human thymus from stem cells,” said Dr. Maehr. “Few labs around the world are working on this but it could provide insight into various immune dysfunctions and possibly, in the long run, to immune engineering strategies to combat immune syndromes, cancer and aging-related decline of immune function.”
Scientists at the UMass Diabetes Center of Excellence now aim to study interaction of thymic cells with human T cells inside of our unique humanized mouse models.
“If we can recreate the thymus defects that can occur in people with T1D, it will allow immunologists to study the process to understand how and why the autoimmune attack occurs,” said Magaletta. “When the root of the problem is identified, therapeutic modifications can be strategized and tested.”
Related Articles
Developing a Thymus in the Maehr Lab to Improve Type 1 Diabetes Research
The Maehr Lab's Thymus Development Study Featured as the Cover Story in Immunity
