Vascular Research
Our Division is committed to the development and integration of
|
Basic Science Research
The Messina Vascular Research Laboratory, in its current form, was established by Dr. Louis Messina in 2006. He has enjoyed continuous extrinsic NIH funding for twenty years. His current research program has two major areas of focus. One is on NIH-funded work to identify the cellular and molecular mechanisms that regulate collateral artery enlargement. His lab has shown that collateral artery enlargement is dependent on both hematopoietic and mesenchymal stem cells rather than as traditionally thought local tissue response by terminal differentiated cells in an ischemic environment. They have shown that oxidant stress by cardiovascular risk factors such as type-2 diabetes and hypercholesterolemia induce epigenetic changes that decreases the number and phenotype of terminally differentiated cells central to collateral artery enlargement such as monocytes. These epigenetic changes induce a higher proportion of pro-inflammatory monocytes than pro-angiogenic monocytes. These findings are generalizable.
The second area of focus is how these effects of oxidant stress on hematopoietic and mesenchymal stem cells affect immunosurveillance against colorectal cancer and wound healing. The overarching result of these studies is that oxidant stress of stem cells is a critical regulator of innate immunity and inflammation. Three NIH RO-1 grants have been submitted this year.
Recent Publications 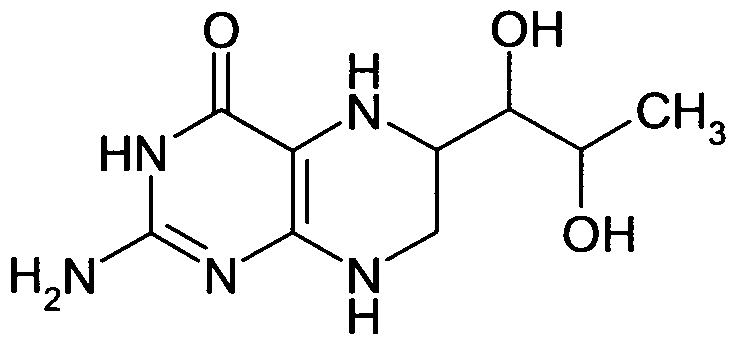
Uncoupling Protein 2 Impacts Endothelial Phenotype via p53-Mediated Control of Mitochondrial Dynamics. Shimasaki Y, Pan N, Messina LM, Li C, Chen K, Liu L, Cooper MP, Vita JA, Keaney JF. Circ Res. 2013 Jul 2. PMID: 23819990
Mesenchymal stem cells as a treatment for peripheral arterial disease: current status and potential impact of type II diabetes on their therapeutic efficacy. Yan J, Tie G, Xu TY, Cecchini K, Messina LM. Stem Cell Rev. 2013 Jun;9(3):360-72. doi: 10.1007/s12015-013-9433-8. PMID: 23475434
Type 2 diabetes restricts multipotency of mesenchymal stem cells and impairs their capacity to augment postischemic neovascularization in db/db mice. Yan J, Tie G, Wang S, Messina KE, DiDato S, Guo S, Messina LM. J Am Heart Assoc. 2012 Dec;1(6):e002238. doi: 10.1161/JAHA.112.002238. Epub 2012 Dec 19. PMID: 23316315
Tetrahydrobiopterin, L-arginine and vitamin C act synergistically to decrease oxidant stress and increase nitric oxide that increases blood flow recovery after hindlimb ischemia in the rat. Yan J, Tie G, Messina LM. Mol Med. 2012 Oct 24;18:1221-30. doi: 10.2119/molmed.2011.00103.revised. PMID: 23212846
Clinical Trials
In collaboration with the Department of Surgery’s Center for Clinical Research and Trials, we are pleased to participate in a number of clinical trials. This allows us to offer cutting edge therapies for a number of vascular conditions including complex aortic pathology, nonreconstructible critical limb ischemia, and hemodialysis access surgery for end-stage renal disease. Sponsorship of these trials is diverse, including the investigator, the National Institutes of Health, the Society for Vascular Surgery, and a number of industry leaders.
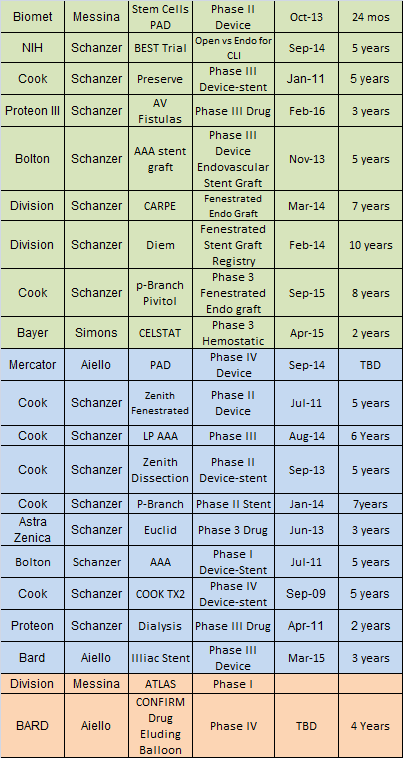

Clinical Outcomes Research
Our group has diverse areas of interest in clinical research, ranging from predictors of sac enlargement after endovascular aneurysm repair, national and regional variation in diagnosis and management of carotid disease, and risk stratification for lower extremity bypass and abdominal aortic aneurysm repair, to research on surgical education and training paradigms. Below is a list of recent peer-reviewed publications and descriptions of specific faculty research interests.
Faculty
Francesco A. Aiello, MD - recent publications
My research program has focused on the evaluation of the accuracy of the two main registries of vascular surgery procedures, the Vascular Surgery Quality Initiative and NISQP. With colleagues from QHS including Drs. Amy Rosen and Bruce Barton, we have to our surprise that neither registry provides an accurate measure of the outcome of vascular surgery procedures. Such registries are fundamental to determining the quality and effectiveness of such procedures. This work has been accepted for presentation at the New England Vascular Society of Vascular Surgery in September and we plan submission of a manuscript to the Journal of Vascular Surgery. In ongoing work, we analyze this data to refine this analysis to shed more light on the sources of this inaccuracy. We hope to transition this work towards preliminary data for a K award.
Louis M. Messina, MD - recent publications
My current research program has two major areas of focus. One is on NIH-funded work to identify the cellular and molecular mechanisms that regulate collateral artery enlargement. We have shown that collateral artery enlargement is dependent on both hematopoietic and mesenchymal stem cells rather than as traditionally thought local tissue response by terminal differentiated cells in an ischemic environment. We have shown that oxidant stress by cardiovascular risk factors such as type-2 diabetes and hypercholesterolemia induce epigenetic changes that decreases the number and phenotype of terminally differentiated cells central to collateral artery enlargement such as monocytes. These epigenetic changes induce a higher proportion of pro-inflammatory monocytes than pro-angiogenic monocytes. These findings are generalizable. The second area of focus is how these effects of oxidant stress on hematopoietic and mesenchymal stem cells affect immunosurveillance against colorectal cancer and wound healing. The overarching result of these studies is that oxidant stress of stem cells is a critical regulator of innate immunity and inflammation. We plan to submit three NIH RO-1 grants this year.
Tammy T. Nguyen, MD, PhD - recent publications
Dr. Tammy Nguyen’s research will focus on developing technologies to enhance wound healing, tissue perfusion and limb salvage. Specifically, she plans to leverage her training as a vascular surgeon (MD) and molecular biologist (PhD) to investigate the molecular mechanisms that compromise the immune system and inhibit wound healing in diabetes and limb threatening ischemia. Type 2 diabetes and atherosclerosis both present with innate immune dysfunction leading to reduced hematopoietic stem cell (HSC) mobilization and dysregulated differentiation towards monocytes-macrophages that are responsible for impaired diabetic wound healing. Currently, the mechanism to explain how diabetes leads to impaired HSC-mediated wound healing in patients is not completely understood. Using patient bone marrow and tissue samples, Dr. Nguyen will study the signaling pathways between adipocytes and HSC-mediated immune function that impairs wound healing in diabetes. Working closely with Drs. Messina (Vascular Surgery) and Corvera (Molecular Medicine), the goal of the lab is to accelerate understanding of non-healing diabetic foot ulcers and provide insight into therapeutic strategies by using patient samples to build a bedside-to-bench and bench-to-bedside translational research model.
Andres Schanzer, MD - recent publications
I  have continued to pursue two major areas of research: the investigation of vascular surgery patient characteristics that predict major clinical outcomes using risk prediction modeling, and the evaluation of new endovascular technologies for the treatment of complex aortic pathology. I also continue to enjoy mentoring our vascular surgery residents on a number of clinical outcomes research projects with the goal of ensuring that each trainee is involved with the design, implementation, presentation, and publication of a clinical study. My primary focus at this time is running our FDA approved physician sponsored investigational device exemption trial evaluating the safety and efficacy of custom made devices and physician modified endografts for the minimally invasive treatment of juxtarenal, pararenal, and thoracoabdominal aortic aneurysms (CARPE-CMD study). We are currently enrolling patients and have been receiving a growing number of regional referrals to the trial. Further details can be found on our Center for Complex Aortic Disease website.
have continued to pursue two major areas of research: the investigation of vascular surgery patient characteristics that predict major clinical outcomes using risk prediction modeling, and the evaluation of new endovascular technologies for the treatment of complex aortic pathology. I also continue to enjoy mentoring our vascular surgery residents on a number of clinical outcomes research projects with the goal of ensuring that each trainee is involved with the design, implementation, presentation, and publication of a clinical study. My primary focus at this time is running our FDA approved physician sponsored investigational device exemption trial evaluating the safety and efficacy of custom made devices and physician modified endografts for the minimally invasive treatment of juxtarenal, pararenal, and thoracoabdominal aortic aneurysms (CARPE-CMD study). We are currently enrolling patients and have been receiving a growing number of regional referrals to the trial. Further details can be found on our Center for Complex Aortic Disease website.
Jessica P. Simons, MD, MPH - recent publications
The emphasis of my research is on methods of assessment of outcomes and quality for vascular disease. Specific current efforts are focused on lower extremity peripheral arterial disease, including a recent publication on the derivation and validation of a risk stratification model for amputation-free survival in patients undergoing lower extremity bypass for CLI. It has been adopted for benchmarking by the Vascular Quality Initiative. I am also working to establish patient-reported outcomes as quality measures for claudication, with the intention that may ultimately be routinely collected as part of larger registry efforts to establish the value of care that we provide.