HCV NS3/4A Protease Inhibitors
Synthesis and SAR Studies of HCV NS3/4A Protease Inhibitors
We have applied the substrate envelope approach to decipher drug resistance against HCV NS3/4A protease inhibitors. We have discovered that, similar to HIV protease inhibitors, drug resistance against HCV NS3/4A inhibitors is defined by the balance of substrate recognition versus inhibitor binding. These findings have led to the development of a substrate envelope model for HCV NS3/4A protease, which we have used to understand the molecular basis of drug resistance against NS3/4A protease inhibitors. We are currently engaged in exploiting the HCV NS3/4A substrate envelope model to rationally design more robust protease inhibitors that are less susceptible to resistance.
The available antiviral therapies, which include PEGylated interferon, ribavirin, and one of the HCV NS3/4A protease inhibitors telaprevir or boceprevir, are ineffective for some patients and cause severe side effects. More potent NS3/4A protease inhibitors are in clinical development, but the long-term effectiveness of these drugs is challenged by the development of drug resistance. Recently, we investigated the role of macrocycles in the susceptibility of NS3/4A protease inhibitors to drug resistance in asunaprevir, danoprevir, vaniprevir, and MK-5172, with similar core structures but varied P2 moieties and macrocyclizations. Linear and macrocyclic analogues of these drugs were designed, synthesized, and tested against wild-type and drug-resistant variants R155K, V36M/R155K, A156T, and D168A in enzymatic and antiviral assays. Macrocyclic inhibitors were generally more potent, but the location of the macrocycle was critical for retaining activity against drug-resistant variants: the P1–P3 macrocyclic inhibitors were less susceptible to drug resistance than the linear and P2–P4 macrocyclic analogues. In addition, the heterocyclic moiety at P2 largely determined the inhibitor resistance profile, susceptibility to drug resistance, and the extent of modulation by the helicase domain. Our findings suggest that to design robust inhibitors that retain potency to drug-resistant NS3/4A protease variants, inhibitors should combine P1–P3 macrocycles with flexible P2 moieties that optimally contact with the invariable catalytic triad of this enzyme (Ali et al., 2013).
gfg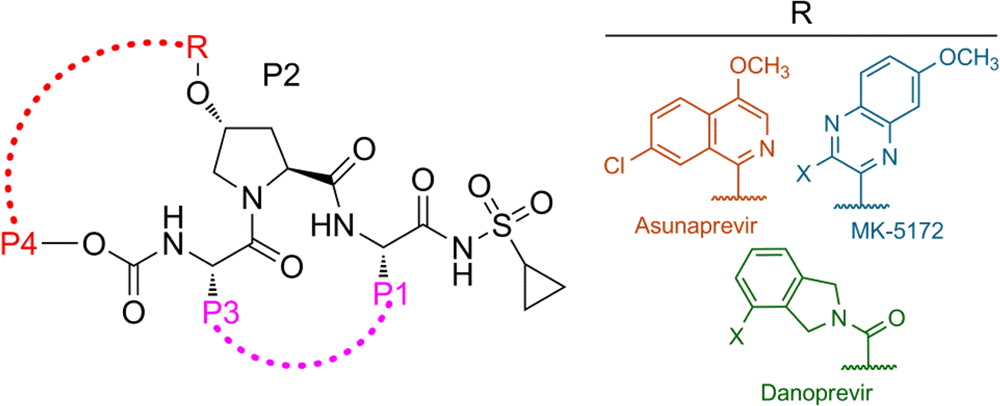 |
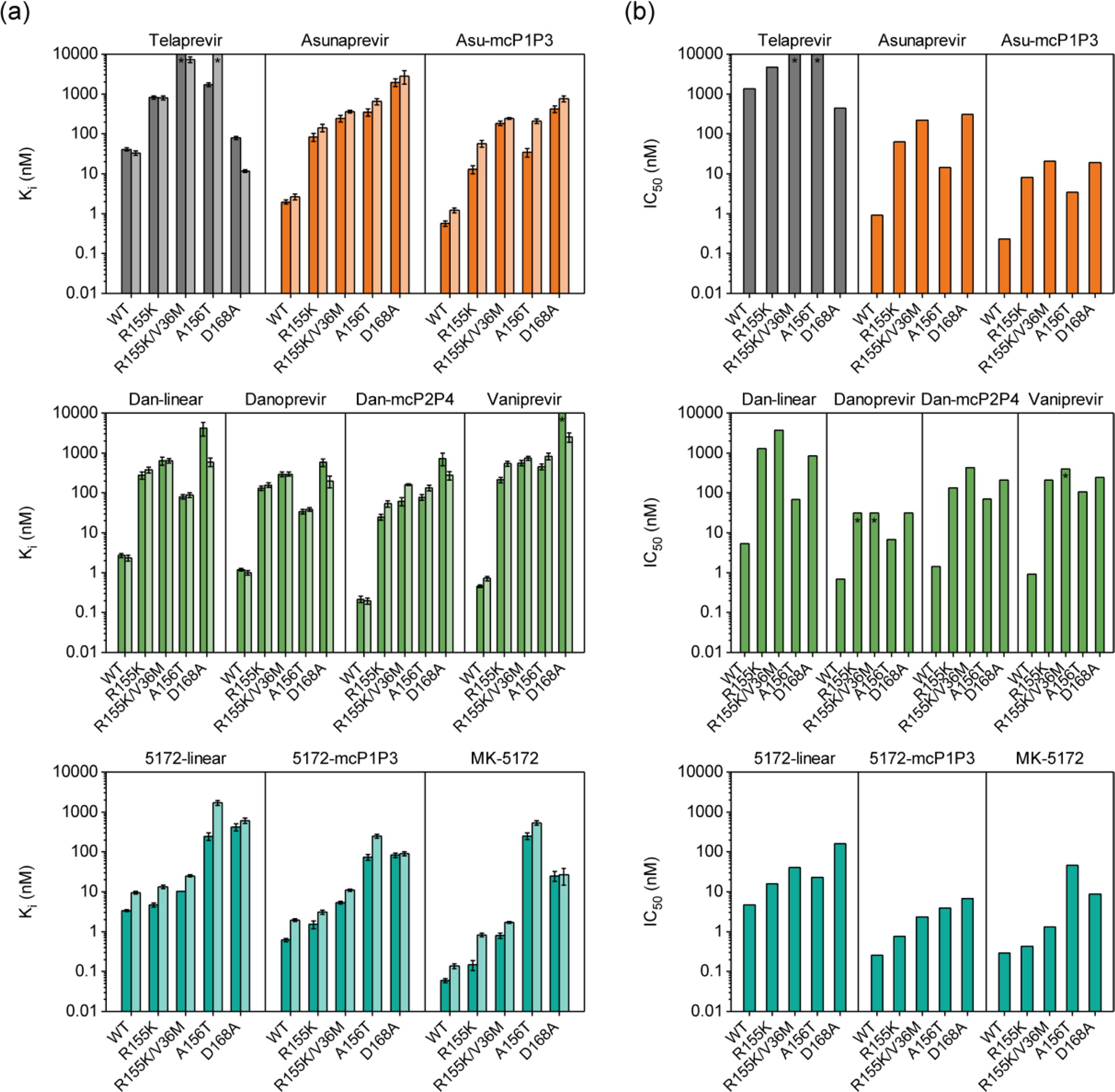 |
| Enzyme inhibition constants for HCV genotype 1a full-length NS3/4A and isolated protease domain (dark and light bars) and (b) replicon-based half maximal inhibitory concentrations for genotype 1b HCV NS3/4A and drug-resistant variants for (first row) telaprevir and asunaprevir series, (second row) danoprevir/vaniprevir series, and (third row) MK-5172 series. Error bars represent standard errors of the mean (n = 4); * indicates Ki and IC50 values are greater than the highest inhibitor concentration tested (Ali et al., 2013). |
|

